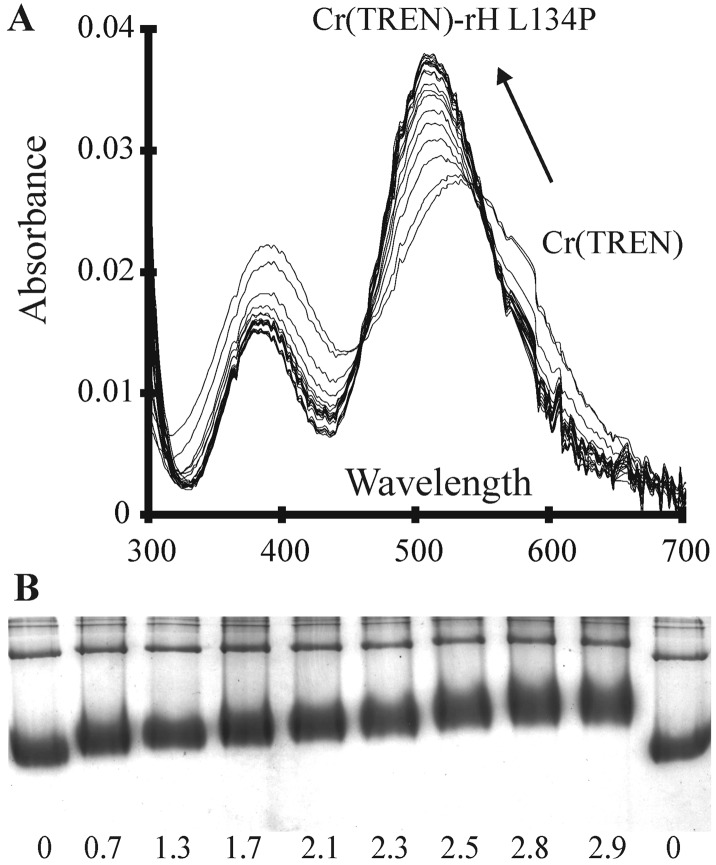
Figure 2.
Cr(TREN)–ferritin Interactions. (A) The effect of ferritin on the Cr(TREN) spectrum. UV-vis spectra showing the shift in the Cr(TREN) d-d bands on complexation to rH-L134P, with two apparent isosbestic points at 544 and 460 nm. Cr(TREN) (0.4 mM, 2 equivalents per subunit) was incubated with rH-L134P (8 μM) at 25°C. Spectra were taken at 20-min intervals. The absorption spectrum of the protein in buffer, before Cr(TREN) addition, was used as a reference. (B) The effect of Cr(TREN) on protein surface charge (electrophoretic mobility). Migration of rH-L134P, with varying amounts of bound Cr(TREN) during electrophoresis in native polyacrylamide gels (6%). Values shown are Cr(TREN) per subunit after removing unbound Cr(TREN) by dialysis.