Abstract
Background
Peripheral arterial disease (PAD) is common and is a marker of systemic atherosclerosis. Patients with symptoms of intermittent claudication (IC) are at increased risk of cardiovascular events (myocardial infarction (MI) and stroke) and of both cardiovascular and all cause mortality.
Objectives
To determine the effectiveness of antiplatelet agents in reducing mortality (all cause and cardiovascular) and cardiovascular events in patients with intermittent claudication.
Search methods
The Cochrane Peripheral Vascular Diseases group searched their Specialised Register (last searched April 2011) and CENTRAL (2011, Issue 2) for publications on antiplatelet agents and IC. In addition reference lists of relevant articles were also searched.
Selection criteria
Double‐blind randomised controlled trials comparing oral antiplatelet agents versus placebo, or versus other antiplatelet agents in patients with stable intermittent claudication were included. Patients with asymptomatic PAD (stage I Fontaine), stage III and IV Fontaine PAD, and those undergoing or awaiting endovascular or surgical intervention were excluded.
Data collection and analysis
Data on methodological quality, participants, interventions and outcomes including all cause mortality, cardiovascular mortality, cardiovascular events, adverse events, pain free walking distance, need for revascularisation, limb amputation and ankle brachial pressure indices were collected. For each outcome, the pooled risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI) was calculated.
Main results
A total of 12 studies with a combined total of 12,168 patients were included in this review. Antiplatelet agents reduced all cause (RR 0.76, 95% CI 0.60 to 0.98) and cardiovascular mortality (RR 0.54, 95% CI 0.32 to 0.93) in patients with IC compared with placebo. A reduction in total cardiovascular events was not statistically significant (RR 0.80, 95% CI 0.63 to 1.01). Data from two trials (which tested clopidogrel and picotamide respectively against aspirin) showed a significantly lower risk of all cause mortality (RR 0.73, 95% CI 0.58 to 0.93) and cardiovascular events (RR 0.81, 95% CI 0.67 to 0.98) with antiplatelets other than aspirin compared with aspirin. Antiplatelet therapy was associated with a higher risk of adverse events, including gastrointestinal symptoms (dyspepsia) (RR 2.11, 95% CI 1.23 to 3.61) and adverse events leading to cessation of therapy (RR 2.05, 95% CI 1.53 to 2.75) compared with placebo; data on major bleeding (RR 1.73, 95% CI 0.51, 5.83) and on adverse events in trials of aspirin versus alternative antiplatelet were limited. Risk of limb deterioration leading to revascularisation was significantly reduced by antiplatelet treatment compared with placebo (RR 0.65, 95% CI 0.43 to 0.97).
Authors' conclusions
Antiplatelet agents have a beneficial effect in reducing all cause mortality and fatal cardiovascular events in patients with IC. Treatment with antiplatelet agents in this patient group however is associated with an increase in adverse effects, including GI symptoms, and healthcare professionals and patients need to be aware of the potential harm as well as the benefit of therapy; more data are required on the effect of antiplatelets on major bleeding. Evidence on the effectiveness of aspirin versus either placebo or an alternative antiplatelet agent is lacking. Evidence for thienopyridine antiplatelet agents was particularly compelling and there is an urgent need for multicentre trials to compare the effects of aspirin against thienopyridines.
Keywords: Humans, Cause of Death, Intermittent Claudication, Intermittent Claudication/drug therapy, Intermittent Claudication/mortality, Myocardial Infarction, Myocardial Infarction/mortality, Myocardial Infarction/prevention & control, Platelet Aggregation Inhibitors, Platelet Aggregation Inhibitors/adverse effects, Platelet Aggregation Inhibitors/therapeutic use, Randomized Controlled Trials as Topic, Stroke, Stroke/mortality, Stroke/prevention & control
Plain language summary
Antiplatelet agents for reducing risks in patients with peripheral arterial disease and cramping pain in legs on walking
Peripheral arterial disease (PAD) refers to the blocking of large arteries. Patients with peripheral arterial disease as a result of narrowing of the arteries in the legs may present with cramping pain in the legs or buttocks on walking; this is also known as intermittent claudication (IC). This group of patients are at high risk of a heart attack, stroke and death. Treatment often involves stopping cigarette smoking and the optimising of other risk factors such as diabetes, high blood pressure and cholesterol. Another treatment often used to reduce the risk of heart attacks and strokes in patients with IC is antiplatelet treatment. Antiplatelets make the blood less sticky and therefore block the formation of blood clots, thereby preventing blockages in arteries which can cause heart attacks and strokes. Antiplatelet treatments include drugs such as aspirin, clopidogrel and dipyridamole, but there is limited evidence to date on the benefits of antiplatelet therapy in patients with IC. Twelve studies with a total of 12,168 patients were included in this review. The analyses show that, in patients with IC, antiplatelet agents reduced the risk of death from all causes, and from heart attack and stroke combined when compared with placebo. When aspirin was compared with other antiplatelet agents, there was some evidence that the alternative antiplatelet had a more beneficial effect in reducing all cause mortality or of suffering a cardiovascular event such as heart attack or stroke. However, this was based on only two trials. Antiplatelet usage, however, does increase the risk of indigestion and may also increase risk of major bleeding events. Despite its widespread use, the evidence for first line use of aspirin in patients with IC is weak and further research is required to determine whether aspirin would be better replaced by a different class of antiplatelet agent which has a greater beneficial effect with fewer side‐effects.
Background
Description of the condition
Atherosclerotic peripheral arterial disease (PAD) of the lower limb is common. In the Edinburgh Artery Study of men and women aged 55 to 74 years, 4.5% had symptomatic PAD. A further 8% had evidence of major asymptomatic disease and 16.6% had abnormal haemodynamic parameters suggesting minor PAD (Fowkes 1991). Epidemiological studies have shown that the incidence of PAD increases with age (Fowkes 1991; Selvin 2004) and is more common in men (Fowkes 2008). One of the commonest non‐invasive methods of diagnosing PAD is the ankle brachial pressure index (ABPI). An ABPI < 0.9 is 95% sensitive and 100% specific for detecting angiogram positive disease (Norgren 2007). Peripheral arterial disease encompasses a spectrum of disease severity. In its early stages, PAD may be asymptomatic. The most common symptom, intermittent claudication (IC), occurs in approximately 0.5 to 14% of the population depending on age, sex and geographical location (Balkau 1994; Fowkes 1991). People with IC experience pain in the calves, thighs or buttocks when they are walking and the pain subsides with rest. At the other extreme of the disease, PAD may manifest as rest pain, ulceration or gangrene.
Many patients with IC have relatively mild complaints, but up to 20% of patients will go on to require reconstructive surgery, and 1 to 2% will eventually undergo amputation (Kannel 1970; Leng 1993). Patients with symptomatic IC can have reduced levels of mobility and quality of life which equate to that associated with some cancers (Belch 2003). Peripheral arterial disease is also a marker of systemic atherosclerosis, and is associated with both concomitant and subsequent coronary and cerebrovascular disease (Aronow 1994; Newman 1993). Compared with age‐matched controls, patients with IC have a three to six‐fold increase in cardiovascular mortality (Criqui 1992; Leng 1996). In the Reduction of Atherothrombosis for Continued Health (REACH) multi‐national registry of patients with known cardiovascular disease or increased cardiovascular risk, the highest cardiovascular event rate was in patients with PAD. At one year follow‐up, 18.2% of PAD patients had suffered a cardiovascular death, myocardial infarction (MI), or stroke, or had been hospitalised for a cardiovascular event compared with 13.3% of the coronary artery disease group and 10% of the cerebrovascular disease group (Steg 2007).
The majority of patients with IC are treated conservatively, including smoking cessation and regular exercise (Gardner 1995; Watson 2008) to improve the collateral blood vessels supplying the lower limbs. Smoking is a particularly important modifiable risk factor for PAD: smokers with PAD are more likely to progress to critical limb ischaemia and to require an amputation or vascular intervention (Jonason 1985) and smoking cessation can reduce overall cardiovascular risk to the level of non‐smokers within two to four years (Rosenberg 1990). Diabetes mellitus is also an important risk factor for the development of PAD. People with diabetes have a 1.5 to 2.5 fold increased risk of symptomatic and asymptomatic PAD compared with non‐diabetics, and the life‐time risk of a lower limb amputation is increased 10 to 16 times (Al‐Delaimy 2004; MacGregor 1999). To date, antihypertensive agents (Singer 2008) and lipid‐lowering therapy (Aung 2007; HPSCG 2007; Meade 2002) have been shown to reduce cardiovascular mortality and morbidity in patients with PAD.
Description of the intervention
Platelets play a key role in haemostasis and in the pathogenesis of atherosclerotic disease. Circulating platelets are quiescent and do not bind to the intact endothelium. However, endothelial damage following injury leads to exposure of circulating platelets to the subendothelial extracellular matrix and triggers platelet recruitment and adhesion (Varga‐Szabo 2008). Platelet adhesion occurs when the von Willebrand factor binds to various glycoprotein (GP) receptors (GP Ib‐IX‐V complex); receptors for collagen and fibronectin also participate in platelet adhesion. Adherent platelets secrete preformed adenosine diphosphate (ADP), fibrinogen, thromboxane A2 (TXA2), and other chemotaxins; clotting factors; and vasoconstrictors from granules, which activate the platelets (Hiatt 2002b). Activated platelets change their shape and secrete ADP and platelet‐derived growth factor, fibrinogen and TXA2. Platelet aggregation involves recruitment of additional platelets and is mediated by the GPIIb/IIIa receptor. This forms a prothrombotic surface that promotes clot formation, contributing to the fibrous plaque development and the consequent thromboembolic complications. The latter can lead to myocardial infarction, stroke or peripheral vascular occlusions (Davi 2007). There is evidence to suggest that PAD is associated with a hypercoagulable state. Blood viscosity, for example, is increased in with patients with claudication (Fagher 1993). Antiplatelet agents, such as aspirin, dipyridamole, picotamide, ticlodipine and clopidogrel act at specific sites of platelet activation and affect multiple pathways. Aspirin (a cyclo‐oxygenase inhibitor) interferes with the biosynthesis of TXA2, prostacyclin and other prostaglandins (Awtry 2000). Dipyridamole (a phosphodiesterase inhibitor) is a platelet adhesion inhibitor that exerts its effects by inhibiting the phosphodiesterase and blocking the uptake of adenosine (Patrono 2004). Picotamide on the other hand interferes with arachidonic acid cascade of platelets by inhibiting TXA2 synthase and antagonising TXA2 receptors (Ratti 1998). Unlike aspirin, picotamide does not affect cyclo‐oxygenase. Ticlopidine and clopidogrel (thienopyridines) interfere with ADP‐mediated platelet activation and aggregation and cause an irreversible non‐competitive inhibition of platelet function (Schror 2000).
Why it is important to do this review
Current use of antiplatelet treatment to reduce the risk of cerebrovascular and coronary events in high risk patients subgroups is based to a great extent on evidence inferred from a meta‐analysis of randomised controlled trials involving patients with coronary and cerebrovascular disease (ATC 2002). The benefit of antiplatelet agents specifically in patients with IC is unknown. In addition, use of antiplatelet agents can be associated with an increased risk of gastrointestinal ulcer and haemorrhage and other adverse events, such as blood dyscrasias (neutropaenia and thrombocytopaenia). There is currently no clear evidence on the risk‐benefit ratio of antiplatelet therapy in people with IC. Novel antiplatelet agents with potentially increased effectiveness and fewer side‐effects compared with aspirin and other older antiplatelet agents are being developed (Ueno 2011), however, there is a paucity of evidence comparing the effectiveness of one antiplatelet agent over another.
Objectives
The primary aim of this review was to determine the effectiveness of antiplatelet agents in reducing mortality (all cause and cardiovascular), and cardiovascular events (fatal and non‐fatal myocardial infarction or stroke) in patients with stable intermittent claudication (Fontaine stage II) (Fontaine 1954).
In addition, the efficacy of antiplatelet agents in reducing the need for revascularisation of the lower limbs and preventing amputation; improving treadmill walking distance; and improving ankle brachial pressure index was assessed. Adverse effects (in particular, major bleeding, gastrointestinal side‐effects and side‐effects leading to cessation of treatment) were also assessed. The efficacy of one or more antiplatelet agent(s) versus placebo or versus an alternative antiplatelet agent was considered.
Methods
Criteria for considering studies for this review
Types of studies
We included all double‐blinded, randomised controlled trials (RCTs) comparing oral antiplatelet agents versus control, or versus other antiplatelet agents in patients with stable IC (Fontaine stage II) (Fontaine 1954). We excluded quasi‐RCTs, single‐blinded or open studies. Quasi‐RCTs are studies randomised by methods of allocation such as alternation, date of birth or case record number (Higgins 2011). We excluded cross‐over trials because we considered them inappropriate for antiplatelet agent treatment in this study population. Minimum duration of follow‐up for trials to be considered for the review was 24 weeks.
Types of participants
We included all patients presenting with stable IC (Fontaine stage II) for more than six months. We excluded studies which included patients with asymptomatic PAD, rest pain, ischaemic ulcer or gangrene (Fontaine stages I, III and IV). Similarly, we excluded patients undergoing planned future vascular surgical intervention (angioplasty, surgery or amputation).
Types of interventions
We considered trials which compared either single or combination oral antiplatelet agents (aspirin, indobufen, sulphinpyrazone, dipyridamole, picotamide, clopidogrel, ticlopidine, prasugrel, ticagrelor, elinogrel, atopaxar and vorapaxar) with placebo or other antiplatelet agent . The intervention must have been given for at least three months. We recorded the type of therapy, dosage, time of starting and duration of therapy. We excluded trials comparing antiplatelets with exercise, anticoagulants or surgery (angioplasty or bypass). We also did not consider studies incorporating vasodilating agents (beraprost, iloprost, cilostazol, pentoxifylline, suloctidil and trapidil), where the main mechanism of action is not thought to be via their antiplatelet effect.
Types of outcome measures
Primary outcomes
All cause mortality
Cardiovascular mortality (death from stroke or myocardial infarction)
Cardiovascular events (fatal and non‐fatal myocardial infarction or stroke)
Secondary outcomes
Adverse events: major bleeding, GI symptoms (specifically dyspepsia) and adverse events leading to cessation of therapy
Pain free walking distance
Revascularisation (angioplasty or surgical bypass)
Limb amputation
Change in ankle brachial pressure index
All definitions were standardised whenever possible.
Major bleeding was defined as a bleeding event that resulted in one or more of the following:
death;
a decrease in haemoglobin concentration of 2 g/dL or more;
transfusion of at least 2 units of blood;
bleeding from a retroperitoneal, intracranial, or intraocular site;
a serious or life‐threatening clinical event;
need for surgical or medical intervention.
Inconsistent or missing outcome definitions were considered as a source of bias.
Search methods for identification of studies
We applied no language restriction on publications or any restrictions regarding publication status.
Electronic searches
The Cochrane Peripheral Vascular Diseases Group searched their Specialised Register (last searched April 2011) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2) at www.thecochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the Trials Search Co‐ordinator (TSC) and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED; and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases (PVD) Group module in The Cochrane Library (www.thecochranelibrary.com).
The following trial databases were searched by the TSC (April 2011) using the term "intermittent claudication" for details of ongoing and unpublished studies:
World Health Organization International Clinical Trials Registry http://apps.who.int/trialsearch/; ClinicalTrials.gov http://clinicaltrials.gov/; Current Controlled Trials http://www.controlled‐trials.com/.
Searching other resources
We searched the reference lists of articles retrieved by electronic searches for additional citations. We contacted trialists for further information in cases where there were missing data or doubts about whether to include trials in the review.
Data collection and analysis
We performed separate analyses for trials of (1) antiplatelet agent(s) versus placebo and (ii) antiplatelet agent(s) versus an alternative antiplatelet agent.
Selection of studies
Two authors (PW and LYC) independently selected trials for inclusion based on titles and abstract retrieved from the full search. Full text papers were obtained and further screening was performed independently by two authors (PW and LYC) if it was felt that the trial could possibly meet the inclusion criteria. In the event of disagreements, a consensus was reached by discussion and if necessary, a final decision was reached following involvement of a third author (GS).
Data extraction and management
Data extraction from included trials was performed independently by two authors (PW and LYC) and entered into a standardised electronic spreadsheet (Microsoft Excel, for details, see Characteristics of included studies). Data from multiple reports of the same study were extracted directly onto a single data collection form on the spreadsheet. The data collated were then compared and any discrepancies or disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two authors (PW and LYC) independently assessed study eligibility and risk of bias using the following domain‐based evaluation: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The risk of bias was classified as 'low risk', 'unclear risk' or 'high risk', and a rating was provided for each study (and for each outcome, for those risks of biases that were outcome specific).
Measures of treatment effect
We used the statistical package (Review Manager 5.1) provided by the Cochrane Collaboration to analyse data. For dichotomous outcomes, statistical analysis was presented as risk ratio (RR) with 95% confidence interval (CI). We used mean difference (MD) with 95% CI for continuous outcomes.
Unit of analysis issues
Participating individuals in the individually randomised trials were the unit of analysis.
Dealing with missing data
We contacted the principal authors of included studies when necessary to clarify data and to provide missing information.
Assessment of heterogeneity
We based all analyses on the intention‐to‐treat data from individual trials. We assessed trial heterogeneity using Chi2 test of heterogeneity, and I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). Where heterogeneity was identified (P < 0.1 or I2 > 50%), we investigated the reason for heterogeneity. If no apparent reason was found, we conducted a random‐effects meta‐analysis to incorporate heterogeneity among studies. In the absence of heterogeneity, we used a fixed‐effect model.
Assessment of reporting biases
We planned to use asymmetry in funnel plots to assess reporting bias but due to the small number of included trials, the power of this analysis would have been too low to distinguish chance from real asymmetry, and therefore this was not performed.
Data synthesis
Where clinical or statistical heterogeneity existed (P < 0.1 or I2 > 50%), we used a random‐effects model. In the absence of heterogeneity we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis for patients with and without diabetes; this was not possible due to the small number of trials that recruited and stratified patients by diabetic status.
Sensitivity analysis
We planned to perform sensitivity analyses based on the risk of bias if there were studies with very high risk of bias included (that is, with high risk methods of allocation concealment and random sequence generation).
Results
Description of studies
For a detailed description of studies, see Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The combined search yielded 1626 records and out of these we identified 232 potentially suitable titles and abstracts . Following review of these titles and abstracts, two authors (PW and LYC) independently reviewed 197 full text articles, of which 115 were found to potentially meet the inclusion criteria (Figure 1). These were assessed further for inclusion in the review.
1.

Study flow diagram.
Included studies
Twelve trials (published in 17 articles) of antiplatelet therapy in patients with IC were included in the review (ADEP 1993; Arcan 1988; Aukland 1982; Auteri 1995; Balsano 1989; CAPRIE 1996; Coto 1989; DAVID 2004; EMATAP 1993; Signorini 1988; STIMS 1990; Tonnesen 1993).
The breakdown of studies according to the countries in which they were performed was as follows:
One Argentinian (EMATAP 1993);
Five Italian (ADEP 1993; Auteri 1995; Coto 1989; DAVID 2004; Signorini 1988);
One Swedish (STIMS 1990);
One British (Aukland 1982);
Two European (Arcan 1988; Balsano 1989);
Two multinational (CAPRIE 1996; Tonnesen 1993).
A total of 12,168 patients were recruited into the trials. The number of participants in each trial ranged from 40 (Signorini 1988) to 6452 (CAPRIE 1996). The age range of participants was 21 to 80 years of age. One trial recruited male patients only (Aukland 1982). Six trials incorporated patients with previous reconstructive surgery or amputation (ADEP 1993; Aukland 1982; CAPRIE 1996; DAVID 2004; EMATAP 1993; STIMS 1990). Two trials excluded patients with insulin dependent diabetes (Auteri 1995; STIMS 1990), and three trials excluded all patients with diabetes (Coto 1989; Signorini 1988; Tonnesen 1993). One trial specifically recruited patients with both IC and diabetes (DAVID 2004).
Ten trials compared an antiplatelet agent against placebo (ADEP 1993; Arcan 1988; Aukland 1982; Auteri 1995; Balsano 1989; Coto 1989; EMATAP 1993; Signorini 1988; STIMS 1990; Tonnesen 1993), and two trials compared an antiplatelet agent against aspirin (CAPRIE 1996; DAVID 2004). The most common antiplatelet agent tested was ticlopidine (Arcan 1988; Aukland 1982; Balsano 1989; EMATAP 1993; STIMS 1990). The breakdown of antiplatelet agents tested was as follows:
Clopidogrel versus aspirin (CAPRIE 1996);
Indobufen versus placebo (Signorini 1988; Tonnesen 1993);
Picotamide versus placebo (ADEP 1993; Coto 1989) or aspirin (DAVID 2004);
Ticlopidine versus placebo (Arcan 1988; Aukland 1982; Balsano 1989; EMATAP 1993; STIMS 1990);
Triflusal versus placebo (Auteri 1995).
Power calculations were performed in eight trials (ADEP 1993; Arcan 1988; Auteri 1995; CAPRIE 1996; DAVID 2004; EMATAP 1993; STIMS 1990; Tonnesen 1993). Intention‐to‐treat (ITT) analysis was performed in seven trials (ADEP 1993; Arcan 1988; CAPRIE 1996; DAVID 2004; EMATAP 1993; STIMS 1990; Tonnesen 1993).
A placebo run‐in period was used in six trials (ADEP 1993; Arcan 1988; Aukland 1982; Balsano 1989; Coto 1989; Tonnesen 1993).
The length of follow‐up varied between the trials:
24 weeks to 12 months: seven trials (Arcan 1988; Aukland 1982; Auteri 1995; Coto 1989; EMATAP 1993; Signorini 1988; Tonnesen 1993);
13 to 24 months: four trials (ADEP 1993; Balsano 1989; CAPRIE 1996; DAVID 2004);
> 5 years: one trial (STIMS 1990).
In terms of primary outcome events, seven trials (ADEP 1993; Arcan 1988; Balsano 1989; CAPRIE 1996; DAVID 2004; EMATAP 1993; STIMS 1990) reported both all cause and cardiovascular mortality. Cardiovascular events (fatal and non‐fatal MI or stroke) were reported in eight trials (ADEP 1993; Arcan 1988; Balsano 1989; CAPRIE 1996; Coto 1989; DAVID 2004; EMATAP 1993; STIMS 1990).
In terms of adverse events, major bleeding was reported in two trials (Balsano 1989; DAVID 2004) and ten trials reported adverse GI symptoms (ADEP 1993; Arcan 1988; Aukland 1982; Auteri 1995; Balsano 1989; Coto 1989; DAVID 2004; EMATAP 1993; Signorini 1988; Tonnesen 1993). Nine trials reported adverse events leading to early cessation of therapy (ADEP 1993; Arcan 1988; Auteri 1995; Balsano 1989; Coto 1989; DAVID 2004; EMATAP 1993; STIMS 1990; Tonnesen 1993).
For local disease outcomes, walking distance was reported in four trials (Auteri 1995; Coto 1989; Signorini 1988; Tonnesen 1993) and two trials had these data in graphical form (Arcan 1988; Balsano 1989). One of the studies (Auteri 1995) only reported walking distance for the antiplatelet intervention group and noted that there was no statistically significant difference compared with the placebo group.
Six trials reported progression of disease leading to revascularisation (ADEP 1993; Arcan 1988; Aukland 1982; Balsano 1989; EMATAP 1993; STIMS 1990) and there were four trials with data on limb deterioration leading to amputation (ADEP 1993; Balsano 1989; DAVID 2004; STIMS 1990). Data on ABPI were reported in one trial (Coto 1989).
Sources of funding were declared in four trials (ADEP 1993; CAPRIE 1996; DAVID 2004; STIMS 1990).
Excluded studies
Ninety‐eight reports of studies were excluded.
We excluded studies which did not fulfil the inclusion criteria. Reasons for exclusion were:
non‐randomised studies (Fiotti 2003; Randi 1985; Smith 1981);
no control group (Belcaro 1991; Mannucci 1987);
shorter than required treatment duration (Abrahamsen 1974; Davi 1985; Jagroop 2004, Landini 1989; Leo 2007; Mangiafico 2000; Pasqualini 2002);
cross‐over trials (Abrahamsen 1974; Canonico 1991; Davi 1985; Eikelboom 2005; Nenci 1979; Nenci 1982; Novo 1996; Wilhite 2003);
using comparison agents excluded from the review (Adriaensen 1976; Ahn 1992; Allegra 1993; Allegra 1994; Andreozzi 1993; Brass 2006; Castano 1999; Castano 2001; Castano 2003; Castano 2004; Cesarone 1994; Ciocon 1997; Duda 2001; Elam 1998; Gillot 1976; Gregoratti 1982; Gresele 2000; Guan 2003; Hevia 1992; Hiatt 2002; Holm 1984; Hsieh 2009; Jones 1982; Kakkar 1981; Labs 1999; Lievre 1996; Lievre 2000; Mangiafico 2000; Mannarino 1991; Mantero 1983; Marelli 1990; Marrapodi 1994; Miyazaki 2007; Mohler 2003; Norgren 2006; Novo 1996; O'Donnell 2008; O'Donnell 2009; Okadome‐Kenchiro 1992; Panchenko 1997; Pollastri 1991; Regenthal 1991; Rossini 1998; Roztocil 1989; Warfarin 2007);
populations that did not meet inclusion criteria, for example, studies which included patients with planned surgical interventions (Cassar 2005; Cassar 2005a; Creutzig 1994; Ehresmann 1977; Ranke 1992; Ranke 1993; Ranke 1994;Schweizer 2003), or asymptomatic PAD participants (CHARISMA 2009; CLIPS 2007; Fowkes 2010; POPADAD 2008) and stage III and IV Fontaine patients (Fabris 1992; Katsumura 1982; Randi 1985);
ex‐vivo studies (Cassar 2006);
unusual dosing (Giansante 1990) for example, once every third day;
trial proposal (Singer 2006; Tepe 2007).
Studies which fulfilled the inclusion criteria (Baumgartner 1992; Bhatt 2000; Boneu 1996; Destors 1985; Harker 1999; Hawker 1984; Hess 1985; Libretti 1986; Libretti 1986a; Neirotti 1994, Randi 1991, Rudofsky 1987; Schoop 1987; Stiegler 1984; Topol 2000) but did not report on any of the primary or secondary outcomes were excluded. Trials in which results for a subgroup of patients with IC were not available separately were also excluded. One study was excluded because of unclear patient grouping and reporting of results (Catalano 1984).
Risk of bias in included studies
See Figure 2 and Figure 3 for a graphical presentation of the risk of bias. Many studies were conducted in the late 1980s or 1990s. The reporting of the methods section was brief in the study papers, and it was not possible to obtain a record of the full protocol. The lack of details was the main reason for the 'unclear' rating for most studies for selection bias, and selective reporting bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In six trials, the method of randomisation was described or performed by an independent organisation (ADEP 1993; Arcan 1988; CAPRIE 1996; DAVID 2004; EMATAP 1993; STIMS 1990), with a low perceived risk of bias. The randomisation method was unclear in six trials (Aukland 1982; Auteri 1995; Balsano 1989; Coto 1989; Signorini 1988; Tonnesen 1993). Allocation concealment method was not reported in most studies.
Blinding
Apart from two studies (Coto 1989; Signorini 1988), all studies clearly reported that a double‐blinded trial was conducted, and reported the methods used to achieve blinding for investigators and patients. Aside from two additional studies which did not clearly report how investigators measuring the outcomes were blinded to the treatment assignment (STIMS 1990; Tonnesen 1993), all other studies reported the method used to prevent detection bias. Many studies used independent committees blinded to treatment allocation to independently verify and assess outcomes.
Incomplete outcome data
Most studies conducted ITT analyses, and continued follow‐up and reporting of patients who discontinued treatment early, and were therefore classed as low risk of bias. The risk of bias was high for six studies (ADEP 1993; Arcan 1988; Aukland 1982; Balsano 1989; DAVID 2004; Signorini 1988), where the drop out rate was high or the absolute difference in the drop‐out rates were large compared with the primary outcome event rates, or both. The follow‐up period varied between studies and ranged from six months (Arcan 1988; Auteri 1995; Coto 1989; EMATAP 1993; Signorini 1988; Tonnesen 1993) to up to seven years (STIMS 1990).
Selective reporting
Many of the studies were conducted in the late 1980s and 1990s. The protocols for these studies were often not clearly reported. Therefore, the risk of bias was unclear for most studies (Auteri 1995; Balsano 1989; Coto 1989; Signorini 1988) and high for one study (Aukland 1982).
Effects of interventions
Twelve trials that fulfilled the inclusion criteria were included in the review. Five antiplatelet agents were tested (clopidogrel, indobufen, picotamide, ticlopidine and triflusal) in these 12 trials. The effects of antiplatelet agents were considered in two separate categories: antiplatelet agent(s) against placebo and antiplatelet agent(s) against aspirin. The initial protocol (Robless 2003) planned to perform analyses on differences in quality of life (QoL) and cost‐effectiveness between patients who received antiplatelet and control, however, there were no data on these outcomes in the included trials. Neither were any reports of cost‐effectiveness of the different agents found.
1. Antiplatelet agent(s) versus placebo
Four different antiplatelet agents (indobufen, picotamide, ticlopidine and triflusal) were tested against placebo in ten trials (ADEP 1993; Arcan 1988; Aukland 1982; Auteri 1995; Balsano 1989; Coto 1989; EMATAP 1993; Signorini 1988; STIMS 1990; Tonnesen 1993). Overall, 4507 patients were included from these trials (2246 in the antiplatelet group, and 2261 in the placebo group).
Primary outcomes
All cause mortality
All cause mortality was reported in five trials (ADEP 1993; Arcan 1988; Balsano 1989; EMATAP 1993; STIMS 1990). Meta‐analysis showed a statistically significant benefit of antiplatelet agents in reducing all cause mortality (RR 0.76, 95% CI 0.60 to 0.98) (P = 0.03, Analysis 1.1). Combined data from trials of ticlopidine, which showed a statistically significant effect in reducing all cause mortality (RR 0.73, 95% CI 0.56 to 0.96) (P = 0.02), contributed 81.9% of weight to this analysis.
1.1. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 1 All cause mortality.
Cardiovascular mortality
Antiplatelet agents demonstrated a statistically significant benefit in reducing cardiovascular mortality in meta‐analysis of four trials that reported this outcome (Arcan 1988; Balsano 1989; EMATAP 1993; STIMS 1990) (RR 0.54, 95% CI 0.32 to 0.93) (P = 0.03, Analysis 1.2). All four trials used ticlopidine as the drug of intervention.
1.2. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 2 Cardiovascular mortality (fatal stroke or MI).
Cardiovascular events
Data were reported for cardiovascular events (fatal and non‐fatal MI or stroke) in six trials (ADEP 1993; Arcan 1988; Balsano 1989; Coto 1989; EMATAP 1993; STIMS 1990). However, one of these, a small trial (n = 40) with six months of follow‐up and a single angina pectoris event in the treatment group (Coto 1989) was excluded from our meta‐analysis on this outcome since "major cardiovascular event" was not defined and it was unclear whether this was a pre‐planned outcome. In meta‐analysis of the remaining five trials, there was a non‐significant reduction in total cardiovascular events in participants receiving antiplatelet therapy compared with placebo (RR 0.80, 95% CI 0.63 to 1.01) (P = 0.06, Analysis 1.3).
1.3. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 3 Cardiovascular event (fatal and non‐fatal MI or stroke).
We subsequently analysed results for the following sub‐categories of cardiovascular events separately; total MI (fatal and non‐fatal), fatal MI, non‐fatal MI, total stroke (fatal and non‐fatal), fatal stroke and non‐fatal stroke. For total MI, data were available from all five trials contributing data on total cardiovascular events. No statistically significant reduction in total MI with antiplatelet therapy was found (RR 0.84, 95% CI 0.63 to 1.12) (P = 0.24, Analysis 1.4). Subgroup analyses by class of antiplatelet did not reveal evidence of a statistically significant reduction in total MI.
1.4. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 4 Myocardial infarction (fatal and non‐fatal).
For fatal and non‐fatal MI separately, data were available from only four of the five trials contributing data on total MI, since the ADEP 1993 study reported only combined fatal and non‐fatal MI. There were no fatal MIs reported in three of these trials (Arcan 1988; Balsano 1989; EMATAP 1993) and in the remaining trial (STIMS 1990) there was no statistically significant effect of antiplatelet therapy (ticlopidine) on fatal MI (RR 0.57, 95% CI 0.31 to 1.05) (P = 0.07, Analysis 1.5). Meta‐analysis of data from the four trials reporting non‐fatal MI showed no statistically significant difference in non‐fatal MI between antiplatelet and placebo (RR 1.01, 95% CI 0.69 to 1.48) (P = 0.96, Analysis 1.6).
1.5. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 5 Myocardial infarction (fatal).
1.6. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 6 Myocardial infarction (non‐fatal).
None of the five trials that reported on total stroke (fatal and non‐fatal) showed a statistically significant reduction in this outcome with antiplatelet and overall there was no statistically significant difference in total stroke between antiplatelet and placebo groups in the meta‐analysis (RR 0.71, 95% CI 0.45 to 1.13) (P = 0.15, Analysis 1.7). Subgroup analyses by class of antiplatelet did not reveal evidence of a statistically significant reduction in stroke events. Meta‐analyses of 4 trials reporting fatal and non‐fatal stroke separately (one trial (ADEP 1993) only reported total stroke events) also did not show statistically significant differences between antiplatelet and placebo for either fatal stroke (RR 0.33, 95% CI 0.07 to 1.64) (P = 0.18, Analysis 1.8) or non‐fatal stroke (RR 0.74, 95% CI 0.42 to 1.30) (P = 0.30, Analysis 1.9).
1.7. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 7 Stroke (fatal and non‐fatal).
1.8. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 8 Stroke (fatal).
1.9. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 9 Stroke (non‐fatal).
Secondary outcomes
Adverse events
Three main adverse events were considered in this review: major bleeding, GI symptoms (dyspepsia) and adverse events leading to early cessation of treatment. Only two trials (Balsano 1989; STIMS 1990) reported major bleeding adverse events in a total of 838 patients and meta‐analysis showed no statistically significant difference between antiplatelet and placebo (RR 1.73, 95% CI 0.51 to 5.83) (P = 0.38, Analysis 1.10) although the 95% confidence intervals were wide. For the two other adverse events, GI symptoms (dyspepsia) (RR 2.11, 95% CI 1.23 to 3.61) (P = 0.006, Analysis 1.11) and adverse events leading to cessation of therapy (RR 2.05, 95% CI 1.53 to 2.75) (P < 0.00001, Analysis 1.12), there were statistically significant increases in adverse events with antiplatelet therapy. For GI symptoms, subgroup analyses by class of antiplatelet showed a statistically significant increase in symptoms with both indobufen (RR 3.29, 95% CI 1.17 to 9.27) (P = 0.02) and ticlopidine (RR 2.40, 95% CI 1.82 to 3.17) (test of subgroup differences P < 0.00001).
1.10. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 10 Major bleeding.
1.11. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 11 GI symptoms (dyspepsia).
1.12. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 12 Early cessation of treatment.
Other secondary outcomes
Pain free walking distance (PFWD) was reported in three trials (Coto 1989; Signorini 1988; Tonnesen 1993) and meta‐analysis showed a statistically significant increase in PFWD with antiplatelet therapy compared with placebo (MD 78.09, 95% CI 12.24 to 143.95) (P = 0.02, Analysis 1.13). Antiplatelet treatment also significantly reduced the need for revascularisation based on meta‐analysis of five trials (ADEP 1993; Arcan 1988; Aukland 1982; Balsano 1989; EMATAP 1993) (RR 0.65, 95% CI 0.43 to 0.97) (P = 0.03, Analysis 1.14). Amputation events were not significantly different between antiplatelet and placebo groups in meta‐analysis of two trials reporting this outcome (RR 0.84, 95% CI 0.38 to 1.86) (P = 0.67, Analysis 1.15 ). Only one trial (Coto 1989) reported effects of antiplatelet on ABPI (MD 0.04, 95% CI ‐0.00 to 0.08).
1.13. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 13 Pain Free Walking Distance.
1.14. Analysis.
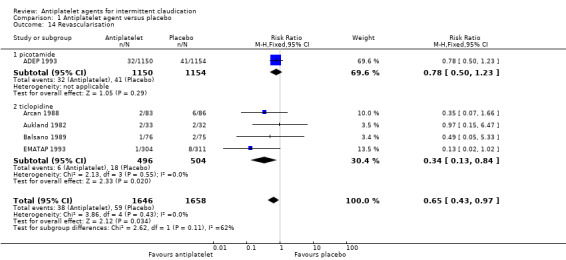
Comparison 1 Antiplatelet agent versus placebo, Outcome 14 Revascularisation.
1.15. Analysis.

Comparison 1 Antiplatelet agent versus placebo, Outcome 15 Limb amputations.
2. Antiplatelet agent(s) versus aspirin
Two different antiplatelet agents (clopidogrel and picotamide) were compared with aspirin in two trials (CAPRIE 1996; DAVID 2004). Overall, 7661 patients were included from these two trials (3826 participants in the antiplatelet group, and 3835 in the aspirin group).
Primary outcomes
All cause mortality and cardiovascular mortality
Compared with aspirin, treatment with an alternative antiplatelet was associated with a significantly lower risk of all cause mortality (RR 0.73, 95% CI 0.58 to 0.93) (P = 0.01, Analysis 2.1). This effect, however, was not reflected in cardiovascular mortality, where there was no statistically significant difference between the alternative antiplatelet and aspirin groups (RR 0.74, 95% CI 0.48 to 1.15) (P = 0.18, Analysis 2.2).
2.1. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 1 All cause mortality.
2.2. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 2 Cardiovascular mortality (fatal stroke or MI).
Cardiovascular events
Meta‐analysis for total cardiovascular events (fatal and non‐fatal MI or stroke) showed a significantly lower risk of events in participants randomised to the alternative antiplatelet compared with aspirin (RR 0.81, 95% CI 0.67 to 0.98) (P = 0.03, Analysis 2.3). This was accounted for mainly by the significantly lower risk of non‐fatal MI with the alternative antiplatelet compared with aspirin (RR 0.65, 95% CI 0.47 to 0.89) (P = 0.008, Analysis 2.6). Total MI events were also significantly lower with the alternative antiplatelet compared with aspirin (RR 0.66, 95% CI 0.50 to 0.86) (P = 0.002, Analysis 2.4). Meta‐analyses for separate cardiovascular events showed no statistically significant difference between the alternative antiplatelet group and the aspirin group for fatal MI (RR 0.68, 95% CI 0.40 to 1.15) (P = 0.15, Analysis 2.5), total stroke (fatal and non‐fatal) (RR 1.01, 95% CI 0.76 to 1.34) (P = 0.93, Analysis 2.7), fatal stroke (RR 0.57, 95% CI 0.05 to 6.44) (P = 0.65, Analysis 2.8) or non‐fatal stroke (RR 1.03, 95% CI 0.76 to 1.39) (P = 0.86, Analysis 2.9).
2.3. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 3 Cardiovascular event (fatal and non‐fatal MI or stroke).
2.6. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 6 Myocardial infarction (non‐fatal).
2.4. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 4 Myocardial infarction (fatal and non‐fatal).
2.5. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 5 Myocardial infarction (fatal).
2.7. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 7 Stroke (fatal and non‐fatal).
2.8. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 8 Stroke (fatal).
2.9. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 9 Stroke (non‐fatal).
Secondary outcomes
Adverse events
Adverse events were not consistently reported by one trial (CAPRIE 1996) that recruited patients with polyvascular aetiology, so inclusion of these data in a meta‐analysis was not possible. The second study (DAVID 2004) reported major bleeding, GI symptoms and adverse events leading to cessation of therapy. Gastrointestinal symptoms (dyspepsia) in this single study were significantly increased in the aspirin group compared with the group receiving picotamide (RR 0.60, 95% CI 0.45 to 0.79) (P = 0.0004, Analysis 2.11). Risks for the other adverse events were not significantly different between the aspirin and picotamide groups (Analysis 2.10; Analysis 2.12).
2.11. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 11 GI symptoms (dyspepsia).
2.10. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 10 Major bleeding.
2.12. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 12 Early cessation of treatment.
Other secondary events
Only one study (DAVID 2004) reported results for limb deterioration leading to amputation and this showed no statistically significant differences in risk ratio between aspirin and picotamide (RR 1.00, 95% CI 0.25 to 4.00) (P = 0.99, Analysis 2.13).
2.13. Analysis.

Comparison 2 Antiplatelet agent(s) versus aspirin, Outcome 13 Amputation.
Discussion
Summary of main results
In this review, antiplatelet treatment was associated with a statistically significant reduction in all cause mortality compared with placebo in patients suffering from intermittent claudication (RR 0.76, 95% CI 0.60 to 0.98). When compared with aspirin, antiplatelet agent(s) other than aspirin were associated with a significantly lower risk of all cause mortality (RR 0.73, 95% CI 0.58 to 0.93).
Cardiovascular mortality was significantly reduced in trial participants treated with antiplatelet compared with placebo (RR 0.54, 95% CI 0.32 to 0.94), but data were only reported on this outcome by trials which used ticlopidine as the drug of intervention. When compared with aspirin, antiplatelet agent(s) other than aspirin were not associated with a significantly lower risk of cardiovascular mortality (RR 0.74, 95% CI 0.48 to 1.15).
Composite cardiovascular events (combined fatal and non‐fatal MI or stroke) appeared lower with antiplatelet treatment compared with placebo (RR 0.80, 95% CI 0.63 to 1.01) but this difference did not reach statistical significance.and there was no statistically significant difference in the risk of individual cardiovascular events i.e. total MI (fatal and non‐fatal), fatal MI, non‐fatal MI, total stroke (fatal and non‐fatal), fatal stroke or non‐fatal stroke, when antiplatelet was compared with placebo. Antiplatelet agent(s) other than aspirin were associated with a significantly lower risk of total cardiovascular events compared with aspirin (RR 0.81, 95% CI 0.67 to 0.98). This was mainly accounted for by the significantly lower risk of MI events (fatal and non‐fatal) (RR 0.66, 95% CI 0.50 to 0.86) and specifically non‐fatal MI (RR 0.65, 95% CI 0.47 to 0.89).
Adverse events (GI symptoms, specifically dyspepsia, and adverse events leading to cessation of therapy) were significantly more common in participants who were treated with antiplatelet agent compared with placebo. The risk of major bleeding was not significantly different between groups treated with antiplatelet and placebo, although data on this outcome were limited. Meta‐analysis for adverse events in trials comparing aspirin with antiplatelet agents other than aspirin was not performed due to lack of data.
Antiplatelet therapy significantly improved pain free walking distance when compared with placebo (MD 78.09, 95% CI 12.24 to 143.95). The risk of revascularisation was also significantly reduced by antiplatelet treatment compared with placebo (RR 0.65, 95% CI 0.43 to 0.97). The risk of amputation was not significantly different between antiplatelet treatment and placebo. Data were only available from one trial comparing antiplatelet agents other than aspirin with aspirin for risk of amputation and this did not show a statistically significant difference.
Overall completeness and applicability of evidence
This review included 12 trials on patients with IC (Fontaine Stage II). Subgroup analyses were performed to assess the effects of varying classes of antiplatelets.
All cause and cardiovascular mortality reporting varied between trials. Composite outcomes, for example "vascular deaths" were used in many studies. Although this has the benefit of reducing the size of the trial required to detect a statistically significant difference, it can reduce our ability to understand the precise impact of antiplatelet use in reducing mortality and morbidity. In addition, not all components within these composite outcomes are of equal importance (Cordoba 2010). For the purpose of this review, cardiovascular mortality was defined as death as a result of MI or stroke, and cardiovascular events was defined as fatal and non‐fatal MI and stroke, these definitions were employed by most of the included studies. ADEP 1993 did not report fatal and non‐fatal events separately and could therefore not be included in meta‐analyses for these individual outcomes.
All cause and cardiovascular mortality reporting was further complicated by the variable lengths of follow‐up. This review was therefore unable to determine a relative risk reduction specifically in relation to treatment length. The majority of the trials (Arcan 1988; Aukland 1982; Auteri 1995; Coto 1989; EMATAP 1993; Signorini 1988; Tonnesen 1993) involved follow‐up for up to a year. For the purpose of this review, cardiovascular mortality was defined as death as a result of myocardial infarction or stroke within 30 days of the event, and this definition was employed by most of the studies. The evidence for cardiovascular mortality should be interpreted as 30‐day risk ratio or relative risk ratio (RRR).
The evidence for aspirin despite its widespread use remains unsubstantiated due to the lack of trials which fulfilled the inclusion criteria specifically for this review. The majority of evidence was derived from historical trials incorporating ticlopidine, which has since been supplanted by another thienopyridine (clopidogrel) due to the increased risk of neutropaenia and thrombocytopaenic purpura (Bennett 1999; Moloney 1993). As a result, ticlopidine is no longer widely used in Europe, the United States of America and Australasia. As thienopyridines, both clopidogrel and ticlopidine are prodrugs that are converted in vivo to pharmacologically active metabolites that inhibit platelet function after irreversibly binding to the platelet ADP P2Y12 receptors. Clopidogrel has been shown to be at least as efficacious as ticlopidine but with better tolerability and fewer side‐effects (Bhatt 2002; Casella 2003; Sudlow 2009). For this reason it is now used in preference to ticlopidine in most countries. We have extrapolated the results of ticlopidine to encompass the thienopyridine group of antiplatelets due to their similar efficacy and mechanism of action. The results of this review and its applicability must therefore be interpreted with this context in mind.
Four large RCTs were excluded (CLIPS 2007; CHARISMA 2009; Fowkes 2010; POPADAD 2008) as they recruited participants with asymptomatic PAD. Both Fowkes 2010 and POPADAD 2008 only included asymptomatic participants.
Further trials were excluded because the study populations included a mixture of Fontaine stage patients and the data by the different Fontaine stages were not available. The review authors propose therefore that for future updates of this review, the review is broadened to include all stages of PAD including stage I (asymptomatic patients).
Quality of the evidence
This review provides a critical, quantitative review of antiplatelet agents in patients with IC. This review examined 12 different trials with 12,168 participants with varying methodology and outcome reporting. Apart from newer studies, most have unclear methods of randomisation and allocation concealment. There is a high rate of early discontinuation of treatment in both arms of the included trials which may potentially affect the final results. These cases were often not clearly reported and it was uncertain whether they were accounted for in studies published in the 1980s and early 1990s.
Potential biases in the review process
This review was limited to RCTs and excluded controlled clinical trials to limit the potential for bias. Most studies were described by their authors as randomised without details being given about the randomisation sequence generation or the type of precautions taken in relation to concealment of allocation. Incomplete outcome data was addressed in the majority of trials.
The main risk of bias from the review process was contributed by the lack of clear definition or variations in the definition of outcomes in the studies reviewed. One example of inconsistencies is that some trials incorporated deaths due to pulmonary embolism and "any deaths that were not clearly non‐vascular" as "vascular deaths". For the purpose of this review, the review authors have strictly employed deaths due to MI and ischaemic stroke to define cardiovascular deaths. Other definitions vary between studies, for example, the definition for major bleeding was not always provided in the studies. Some studies only reported "bleeding", without any further details, while others provided a list of different types of bleeding. For the purpose of this review, a pragmatic approach was taken; the review authors included data which were labelled as "major bleeding", or when there was an attempt to distinguish more serious bleeding events from the minor ones. If data on individual components were available, these were also extracted and added up for the major bleeding outcomes.
In addition, a number of studies were excluded because patients with different stages of PAD (either Fontaine stage I participants or Fontaine stage III and IV patients) were included in the study populations and the results for the different stages were not presented separately.
The risk of data collection bias was reduced by independent data extractions by two review authors, and the use of predefined outcomes in our review protocol. Where there were inconsistencies in the data extracted, these were discussed between the review authors to reach an agreement.
Agreements and disagreements with other studies or reviews
This review demonstrated statistically significant risk reductions in terms of all cause mortality and cardiovascular mortality with antiplatelet treatment in patients with IC. This was consistent with the Antithrombotic Trialists' Collaboration (ATC 2002) report, which similarly showed a proportional reduction of 23% in serious vascular events in patients with PAD who were treated with antiplatelet agents compared with placebo. This review only included patients who had received at least three months of therapy as opposed to four weeks of treatment described in the ATC trial (ATC 2002) therefore reducing the potential of bias from under‐reporting from a short period of treatment, and also allowing real assessment of antiplatelet agents and their longer term impact on mortality.
Basili 2010 reviewed the effects of antiplatelets on the composite outcome of vascular events (non‐fatal MI, non‐fatal stroke of ischaemic or unknown aetiology or vascular death) in patients with IC (surgical series excluded) and found an odds ratio of 0.839 (95% CI 0.73 to 0.97) in favour of antiplatelet treatment compared with placebo. This meta‐analysis from Basili and colleagues (Basili 2010) also found that even though aspirin reduced vascular events, this was not statistically significant.
Another meta‐analysis of aspirin in PAD patients (Berger 2009) also showed no significant benefit of aspirin on cardiovascular events when compared with placebo or control. This review, however, showed that aspirin did accord a significant reduction in the risk of non‐fatal stroke.
Authors' conclusions
Implications for practice.
Antiplatelet agents have proven efficacy in patients with IC in terms of reduction in all cause and cardiovascular mortality. The review was limited to patients with stage II Fontaine and cannot be extrapolated to patients with stage I, III or IV Fontaine, or patients requiring surgical intervention (endovascular treatment, surgical bypass or amputation). The results were highly influenced by trials which used ticlopidine (a thienopyridine) as the intervention antiplatelet, but this agent is no longer available in many countries due to the availability of clopidogrel which has an improved safety profile. When aspirin was compared with other antiplatelet agent(s), there was no evidence that aspirin has any benefit over picotamide or clopidogrel. In fact, there was a reduction in risk of all cause mortality in patients treated with an alternative antiplatelet compared with those in the aspirin group. There is a need to reconsider the use of aspirin as the drug of choice in most clinical practice guidelines in view of the lack of evidence on aspirin compared with the evidence for the thienopyridine class of antiplatelet agents.
Compared with placebo, antiplatelet agents have a significant risk of adverse events (GI side‐effects, specifically dyspepsia and adverse events leading to cessation of therapy) and both healthcare professionals and patients need to be aware of this potential harm. Interestingly, there was no statistically significant difference in the risk of major bleeding between antiplatelet treatment and placebo, but the latter outcome has to be interpreted with caution as it was only reported in only two trials with a relatively small number of participants and the result demonstrated wide 95% confidence intervals.
Implications for research.
The methodology of RCTs in this area of research needs to be better standardised in order to allow for future meta‐analysis. Power calculations should be performed to ensure sufficient patient recruitment. The authors recommend that future trialists should consistently report on all cause and cardiovascular mortality. For the latter outcome, this should be defined as death within 30 days of either MI or ischaemic stroke. Follow‐up length should be increased to at least one year to allow a consistent reporting of risk reduction which would be clinically meaningful. Risk from cancer deaths have always been defined in relation to time i.e. either one‐year, three‐year or five‐year, and it is time similar reporting in patients with IC is performed. Separate reporting of fatal and non‐fatal MI and stroke would be helpful to allow clear distinction of the effects of antiplatelet agents on these morbidities, even when a composite outcome is used as the primary outcome.
There was no consistent definition of major bleeding and the review authors would strongly recommend using the following for future trials:
death due to major bleeding,
a decrease in haemoglobin concentration of 2g/dL or more,
transfusion of at least 2 units of blood,
bleeding from a retroperitoneal, intracranial, or intraocular site,
a serious or life‐threatening clinical event,
requiring surgical or medical intervention.
Due to the paucity of data on use of aspirin and its widespread use in the western world, there is an urgent need for multicentre trials to examine the effects of aspirin in patients with IC.
History
Protocol first published: Issue 1, 1999 Review first published: Issue 11, 2011
| Date | Event | Description |
|---|---|---|
| 3 November 2008 | Amended | Converted to new review format. |
| 13 November 2003 | Amended | A protocol on this topic was originally published in Issue 1, 1999 of The Cochrane Library by Professor David Moher and his team. Mr Peter Robless has now taken over the review and has amended the protocol. |
Notes
August 2003: A protocol on this topic was originally published in Issue 1, 1999 of The Cochrane Library by Professor David Moher and his team. Mr Peter Robless and colleagues have taken over the review.
Acknowledgements
A protocol and draft review of this topic was originally written by a team led by Dr David Moher. The authors would like to thank the personnel from Cochrane Peripheral Vascular Diseases Review group, especially Marlene Stewart and Karen Welch for their invaluable assistance and advice.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor Platelet Aggregation Inhibitors explode all trees | 7499 |
| #2 | MeSH descriptor Platelet Activation explode all trees | 1670 |
| #3 | (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*):ti,ab,kw | 1661 |
| #4 | ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near3 (antagonist or inhibitor)):ti,ab,kw | 5439 |
| #5 | (gp* or glycoprotein* or protease or P2Y12 or TXA2) near3 (inhibit* or antag*):ti,ab,kw | 1654 |
| #6 | (aspirin* or nitroaspirin or ASA or "acetyl salicylic acid*" or "acetylsalicylic acid" or "acetyl‐salicylic acid"):ti,ab,kw | 12752 |
| #7 | (carbasalate calcium or indobufen or triflusal or Disgren or Grendis or Triflux):ti,ab,kw | 158 |
| #8 | (GR144053 or GR‐144053 or abciximab or tirofiban* or eftifibatid or eptifibatide or ReoPro or Integrilin* or Aggrastat ):ti,ab,kw | 832 |
| #9 | (thienopyrid* or thiophen* or clopidogrel or Plavix or Iscover or prasugrel or Effient or ticlopidine or Ticlid or Ticagrelor or Cangrelor or Elinogrel):ti,ab,kw | 2431 |
| #10 | cilostazol or Pletal or d?pyridamol? or Persantin or Triflusal or picotamide:ti,ab,kw | 1442 |
| #11 | (picotinamide or suloctidil or sulphinpyrazone):ti,ab,kw | 83 |
| #12 | (satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151):ti,ab,kw | 13 |
| #13 | (epoprostenol* or iloprost* or ketanserin* or milrinone* or mopidamol*):ti,ab,kw | 1313 |
| #14 | (Dispril or Albyl* or Ticlid* or Persantin* or Plavix or Aggrenox or Plasugrel or Ticagrelor or Cangrelor or SCH 530348 or SCH530348 or Ozagrel or OKY046 or OKY‐046):ti,ab,kw | 182 |
| #15 | E5555 or NCX 4016 or NCX4016 or PRT060128 or PRT 060128 or S18886 or S 18886 | 8 |
| #16 | terutroban | 6 |
| #17 | PRT060128 or PRT 060128 | 0 |
| #18 | AZD6140 or AZD 6140 | 10 |
| #19 | MeSH descriptor Arteriosclerosis, this term only | 902 |
| #20 | MeSH descriptor Arteriolosclerosis, this term only | 0 |
| #21 | MeSH descriptor Arteriosclerosis Obliterans, this term only | 68 |
| #22 | MeSH descriptor Atherosclerosis, this term only | 320 |
| #23 | MeSH descriptor Arterial Occlusive Diseases, this term only | 745 |
| #24 | MeSH descriptor Intermittent Claudication, this term only | 671 |
| #25 | MeSH descriptor Peripheral Vascular Diseases, this term only | 562 |
| #26 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD):ti,ab,kw | 5930 |
| #27 | (arter* or vascular or vein* or veno* or peripher*) near (occlus* or steno* or obstuct* or lesio* or block*):ti,ab,kw | 6218 |
| #28 | (peripheral near3 dis*):ti,ab,kw | 2430 |
| #29 | (claudic* or hinken*):ti,ab,kw | 1211 |
| #30 | (#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29) | 13432 |
| #31 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) | 23377 |
| #32 | (#30 AND #31) | 1625 |
Data and analyses
Comparison 1. Antiplatelet agent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.98] |
| 1.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.66] |
| 1.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.96] |
| 2 Cardiovascular mortality (fatal stroke or MI) | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| 2.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.93] |
| 3 Cardiovascular event (fatal and non‐fatal MI or stroke) | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 3.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.50] |
| 3.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 4 Myocardial infarction (fatal and non‐fatal) | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 4.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.39, 1.69] |
| 4.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 5 Myocardial infarction (fatal) | 4 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.05] |
| 5.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.05] |
| 6 Myocardial infarction (non‐fatal) | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 6.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 7 Stroke (fatal and non‐fatal) | 5 | 3926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.13] |
| 7.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.30] |
| 7.2 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.12] |
| 8 Stroke (fatal) | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.64] |
| 8.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.64] |
| 9 Stroke (non‐fatal) | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.30] |
| 9.1 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.30] |
| 10 Major bleeding | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.51, 5.83] |
| 10.1 ticlopidine | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.51, 5.83] |
| 11 GI symptoms (dyspepsia) | 9 | 3818 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.23, 3.61] |
| 11.1 indobufen | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [1.17, 9.27] |
| 11.2 picotamide | 2 | 2344 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.18] |
| 11.3 ticlopidine | 4 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.82, 3.17] |
| 11.4 trifusal | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 6.41 [0.79, 51.64] |
| 12 Early cessation of treatment | 8 | 4388 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.53, 2.75] |
| 12.1 indobufen | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.47, 4.49] |
| 12.2 picotamide | 2 | 2344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.73, 2.84] |
| 12.3 ticlopidine | 4 | 1622 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.66, 3.31] |
| 12.4 trifusal | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.69] |
| 13 Pain Free Walking Distance | 3 | 329 | Mean Difference (IV, Random, 95% CI) | 78.09 [12.24, 143.95] |
| 13.1 indobufen | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 196.87 [‐85.58, 479.32] |
| 13.2 picotamide | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 38.70 [2.68, 74.72] |
| 14 Revascularisation | 5 | 3304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.43, 0.97] |
| 14.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 14.2 ticlopidine | 4 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.84] |
| 15 Limb amputations | 2 | 2991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 15.1 picotamide | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.98] |
| 15.2 ticlopidine | 1 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.32, 2.35] |
Comparison 2. Antiplatelet agent(s) versus aspirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.93] |
| 1.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.02] |
| 1.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.98] |
| 2 Cardiovascular mortality (fatal stroke or MI) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.15] |
| 2.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.35] |
| 2.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.31] |
| 3 Cardiovascular event (fatal and non‐fatal MI or stroke) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.98] |
| 3.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.97] |
| 3.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.57, 1.54] |
| 4 Myocardial infarction (fatal and non‐fatal) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.86] |
| 4.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.85] |
| 4.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.55] |
| 5 Myocardial infarction (fatal) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.15] |
| 5.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.21] |
| 5.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.25] |
| 6 Myocardial infarction (non‐fatal) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.47, 0.89] |
| 6.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.88] |
| 6.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.36, 1.92] |
| 7 Stroke (fatal and non‐fatal) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 7.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| 7.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 8 Stroke (fatal) | 2 | 7661 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.05, 6.44] |
| 8.1 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.55, 3.42] |
| 8.2 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 9 Stroke (non‐fatal) | 2 | 7661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| 9.1 picotamide vs aspirin | 1 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.74, 4.16] |
| 9.2 clopidogrel vs aspirin | 1 | 6452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.31] |
| 10 Major bleeding | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 GI symptoms (dyspepsia) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Early cessation of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Amputation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 picotamide vs aspirin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ADEP 1993.
| Methods | Randomised, double‐blinded, controlled trial, stratified by centre. Multicentre (120 Italian centres) study. Enrolment started in January 1989. Power calculation: Based on detection of 25% reduction of "major and minor events" compared with untreated patients (estimated as 14% in 18 months from PACK study), the number of patients required would be 1100 for α = 0.05 and β = 0.20, one‐tailed test. The number of patients enrolled increased by 10% to account for lost to follow‐up. ITT analysis: performed. Single‐blinded run‐in period of one month on placebo treatment. Follow‐up length: 18 months. |
|
| Participants | Number of patients randomised: 2304. 1150 (picotamide) versus 1154 (placebo). Male : female ratio = 84.9% male (picotamide); 83.6% male in (placebo). Age (mean with SD): 63.4 ± 7.31 (picotamide); 62.9 ± 7.45 (placebo). Inclusion criteria: consecutive patients up to age of 80 suffering from PVD screened. PVD was defined according to one or more of the following criteria: patients with claudication defined as leg pain on walking that disappeared in five minutes on standing and an ABPI by Doppler ultrasonography ≤0.85 in the posterior and anterior tibial artery of one foot; or patients with claudication with previous amputation or reconstructive vascular surgery. Exclusion criteria: treatment with antiplatelet drugs such as aspirin, ticlopidine, dipyridamole, indobufen and other NSAIDs, anticoagulants; pain at rest; skin lesions; myocardial infarction, stroke, or survival intervention in the previous three months; stable or unstable angina requiring aortocoronary bypass or angioplasty; liver insufficiency (prothrombin activity ≤ 40%); serious renal disorders (serum creatinine ≥2.8 mg%) and other conditions resulting in a life expectancy of < 2 years. |
|
| Interventions | Intervention: picotamide 300 mg tds. Control: placebo tds. |
|
| Outcomes | All cause mortality. Cardiovascular mortality. Myocardial infarction (fatal and non‐fatal). Stroke (fatal and non‐fatal). Adverse events leading to cessation of therapy. Progression of disease resulting in need for revascularisation. Amputation (above ankle). Adverse events: adverse events leading to cessation of therapy, gastrointestinal symptoms. |
|
| Notes | Source of funding: Samil S.p.A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation lists were generated by an automatic procedure developed expressly to have two balanced groups in each centre". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study", "placebo used", "appearance and taste of the capsules were identical". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind study", "qualitative evaluation of selected events validated under blinded conditions by an independent review committee". Comment: it was not stated explicitly if assessors were blinded to the randomisation arm. Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 57/1150(4.9%) (picotamide), 59/1154 (5.1%) (placebo). Withdrawal from study: 48/1150 (4.2%) (picotamide), 29/1154 (2.5%) (placebo). Comment: rate of withdrawal is higher in picotamide group (P = 0.02, Chi2 test). Rate of patients lost to follow‐up were comparable (P = 0.86, Chi2 test). |
| Selective reporting (reporting bias) | Low risk | Outcomes clearly listed and defined, all reported clearly in a table. |
Arcan 1988.
| Methods | Randomised, double blinded, placebo controlled trial. Multicentre (29 French and 1 Swiss) study. Study period: December 1982 ‐ October 1985. Power calculation: 204 patients required, based on a "success" rate of 30% with placebo increased to 50% with ticlopidine. The hypothesis under investigation was set one‐tailed with a type I error of 0.05 and a statistical power of 0.90. ITT analysis: performed. Four week, single‐blind placebo run in. Follow‐up length: 24 weeks. |
|
| Participants | Number of patients: 169. 83 (ticlopidine) versus 86 (placebo). Male : female ratio = 154 : 15. Age (mean): 59.87 ± 1.03 (ticlopidine); 58.53 ± 0.98 (placebo). Inclusion criteria: symptomatic intermittent claudication for at least 12 months and had to present a walking distance assessed by treadmill testing (3.2 km/h, 10% slope, ambient temperature 20‐24 oC) of between 50 and 300 meters. Patient was included if the relative variation of the walking distance was within 25% of initial values at the end of the run‐in period. A recent confirmation of obstructive arterial disease was required by either angiography (< 6 months) or Doppler studies (< 3 months). Exclusion criteria: patient less than 35 or older than 75 years old, stage III or IV Fontaine disease, purely diabetic arteriopathy, vascular surgery within the past six months or planned within the next six months, severe hypertension not adequately controlled by treatment, need for treatment with anti‐inflammatory or anti‐coagulant or vasodilating agents, any contra‐indication to ticlopidine, hepatic or renal disease, and poor vital prognosis. |
|
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo bd. |
|
| Outcomes | All cause mortality. Cardiovascular mortality. Cardiovascular events (MI, stroke or TIA). Walking distance: total and pain free walking distance (data presented in graphical form). Adverse events: adverse events leading to cessation of therapy; GI symptoms (dyspepsia). Need for revascularisation. |
|
| Notes | Trial enrolment terminated early because of persistence of a much slower recruitment rate than expected. Source of funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An academic audit centre...issued the randomisation list". Comment: However, it was unclear how sequence generation was conducted. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An academic audit centre, independent of the sponsor and clinical investigators, was established, to implement an external quality control. It participated in all administrative commitment. It issued the randomisation list". Comment: Unclear how list was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An academic audit centre, independent of the sponsor and clinical investigators...carried out the labelling of the treatment containers", "matching placebo". Comment: most likely participants were blinded to the treatment that they received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "validation committee, blinded to the allocated treatment, had to validate and classify the patients' critical events and evaluate all the files to assign a final result of 'success' or 'failure'". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients discontinued treatment early 17/83 (20.5%) ticlopidine group, 12/86 (14.0%) placebo group, mostly (14/83 (16.9%) ticlopidine, 11/86 (12.8%) placebo) due to occurrence of critical events. Comment: All patients fully accounted for, including those who had early withdrawals of treatment. The numbers were also balanced between arms (P = 0.26, Chi2 test) and events reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to the method stated in the paper, and consistent with objectives of paper (earlier protocol published in French not accessed). |
Aukland 1982.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Single centre (UK) study. Power calculation: not performed. ITT analysis: not performed. Placebo run‐in period of four weeks. Follow‐up length: 12 months. |
|
| Participants | Number of patients enrolled: 65. Number of patients evaluated: 51. 25 (ticlopidine) versus 26 (placebo). All male. Age: Mean 59 (range 40 to 75). Inclusion criteria: intermittent claudication for at least one year, no change in claudication distance for at least three months, no rest pain, and patients not considered for arterial surgery. Exclusion criteria: not specified. |
|
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo. |
|
| Outcomes | Need for revascularisation. Adverse events: GI symptoms. |
|
| Notes | 17 patients had undergone previous vascular reconstructive surgery. 16 patients had history of angina or myocardial infarction. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "one year randomised trial". Comment: randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, one year randomised trial". Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind, one year randomised trial". Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop out rates: 8/33 (24.2%) ticlopidine, 6/32 (19.0%) placebo Comment: drop out rates comparable (P = 0.59, Chi2 test) between groups. four withdrawals, four defaulters, two deaths, and three with non‐thrombotic medical conditions. |
| Selective reporting (reporting bias) | High risk | Did not present ABPI or walking results for placebo group. |
Auteri 1995.
| Methods | Randomised, double blinded, placebo controlled trial. Multicentre (seven Italian centres) study. Power calculation: based on a 5% risk of error of the first type (α) and a 10% risk of error of the second type (β), on the hypothesis of a 60% frequency of success (40% increase of total walking distance over the basal level after 24 weeks of treatment with triflusal) and 30% target difference versus placebo. ITT analysis: not performed. Follow‐up length: 24 weeks. |
|
| Participants | Number of patients: 122. 59 (triflusal) versus 63 (placebo). Male : female ratio = 112 : 16. Age (mean and SD): 64 ± 7.7 (range 40 ‐ 75). Inclusion criteria: stage II Fontaine claudicants. Exclusion criteria: allergy or hypersensitivity to cyclo‐oxygenase inhibitors, assuming drugs that might affect coagulation or fibrinolysis, affected by hypertension, recent myocardial infarction, stroke, cranial trauma or other conditions for active bleeding, peptic ulcers or active organic dyspeptic syndromes, coagulation disorders or other conditions with haemorrhagic, neutropaenia, insulin dependent diabetes, lung, kidney, liver, neurological, psychiatric or haematological disorders or other systemic diseases of clinical importance, any type of neoplasia, surgery during the three antecedent months and use of narcotics. |
|
| Interventions | Intervention: triflusal 300 mg bd. Control: placebo bd. |
|
| Outcomes | Walking distance: total and pain free walking distance. Adverse events: adverse events leading to cessation of therapy, GI symptoms. |
|
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random oral administration". Comment: unclear how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind versus placebo". Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind versus placebo". Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Early cessation of treatment:: 4/59 (6.8%) triflusal group, 1/63 (1.6%) placebo group. Comment: All patients who stopped treatment early were fully accounted for. Drop out rates comparable between groups (P = 0.20, Fisher's exact test). |
| Selective reporting (reporting bias) | Unclear risk | Original study protocol cannot be obtained, but matches methods section. Comment: type of outcomes reported in paper is rational compared with the objectives and length of treatment in the study. |
Balsano 1989.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Multicentre (European) study. Power calculation: not performed. Three month single‐blind placebo run‐in period. ITT analysis: performed. Follow‐up length: 21 months. |
|
| Participants | Number of patients: 151. 76 (ticlopidine) versus 75 (placebo). Male : female ratio = 110 : 42. Age (mean): 59.5 (range 35 ‐ 75) (ticlopidine); 59.9 (range 40 ‐ 75) (placebo). Inclusion criteria: typical intermittent claudication for at least six months, pain free walking distance of 300 m or less as determined on a treadmill (4 kph, no inclination), ankle‐arm systolic blood pressure ratio ≤0.9 at rest in the claudicating leg and further decreased three minutes after the end of the walking test. Exclusion criteria: age > 75 years, treatment in the last six months with angioplasty, thrombolytic drugs or vascular surgery; presence of rest pain or ischaemic skin ulcer; unable to walk on the treadmill, absent ultrasound signals at any foot arteries; concomitant use of oral anti‐coagulants, aspirin, buflomedil, clofibrate, dipyridamole, ditazol, flunarizine, nicergoline, pentoxifylline, suloctidil and sulphinpyrazone. |
|
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo bd. |
|
| Outcomes | All cause mortality. Vascular mortality (stroke or MI related death). Vascular events (fatal and non‐fatal MI or stroke). Walking distance: total and pain free walking distance (data could not be extracted). Revascularisation. Amputation. ABPI: data could not be extracted. Adverse events: adverse events resulting in cessation of study treatment; major bleeding, GI symptoms (dyspepsia). |
|
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned". Comment: randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study", "all tablets were indistinguishable in appearance and taste". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind study". Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate: 17/76 (22.3%) ticlopidine group, 14/75 (18.7%) placebo group. Comment: drop‐out rate comparable between groups (P = 0.57, Chi2 test). Other reasons of drop out were not clear. |
| Selective reporting (reporting bias) | Unclear risk | Comment: type of outcomes reported in paper is rational compared with the objectives and length of treatment in the study. |
CAPRIE 1996.
| Methods | Randomised, double‐blinded, controlled trial Multicentre (384 centres in 16 countries: US, Canada and Europe) study. Power calculation: 5000 patients required in PAD subgroup, assuming a three‐year event rate of 14% for PAD, and 25% for patients with stroke or MI, study was expected to have 90% power to detect an overall relative risk reduction of 11.6%, based on the ITT analysis with a two sided α = 0.05. ITT analysis: performed. Study period: March 1992 to February 1996. Follow‐up length: Varied for patients, common stop date for all. (Mean: 1.91 years). |
|
| Participants | Number of patients: 6452. 3223 (clopidogrel) versus 3229 (aspirin). Male : female ratio = 73% male (clopidogrel); 72% male (aspirin). Age: (mean with SD): 64.2 ± 9.6 (clopidogrel); 64.4 ± 9.7 (aspirin). Inclusion criteria: for PAD patients ‐ intermittent claudication (WHO: leg pain on walking, disappearing in <10 min on standing) of presumed atherosclerotic origin; and ABPI ≤ 0.85 in either leg at rest (two assessments on separate days); or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty with no persisting complications from intervention. Exclusion criteria: age <21 years, scheduled for major surgery, severe co‐morbidity likely to limit life expectancy to less than three years, uncontrolled hypertension, contraindication to study drugs (severe renal or hepatic insufficiency; history of haemostatic disorder or systemic bleeding, thrombocytopaenia or neutropaenia, drug induced haematological or hepatic abnormalities; abnormal WCC, differential or platelet count; anticipated requirement for long term anticoagulant, non‐study antiplatelet drugs or NSAIDs affecting platelet function; history of aspirin sensitivity), women of childbearing age not using reliable contraception, currently receiving investigation drug, previous participation in other clopidogrel studies and geographic or other factors making study participation impractical. |
|
| Interventions | Intervention: clopidogrel 75 mg plus aspirin placebo od. Control: aspirin 325 mg plus clopidogrel placebo od. |
|
| Outcomes | All cause mortality. Vascular mortality. Cardiovascular events (fatal or non‐fatal MI and ischaemic stroke). Vascular events: amputation ‐ results could not be inferred (for PAD). Adverse events: gastrointestinal and intracranial haemorrhage (data for PAD could not be inferred). |
|
| Notes | Source of funding: Sanofi‐Aventis and Bristol‐Myers Squibb. Included patients with ischaemic stroke and myocardial infarction. Data was available for patients with PAD. PAD patients included patients with previous leg amputation, reconstructive surgery or angioplasty with no persisting complications from intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated blocks of four treatments, stratified by disease subgroup and treatment centre". |
| Allocation concealment (selection bias) | Low risk | Quote: "access to the study code restricted to Independent Statistical Centre, Chairman of External Safety and Efficacy Monitoring Committee and two independent companies responsible for preparing the study drug". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients allocated study drugs sequentially from supplies at the clinical centre", "supplies were in identical blister packs containing either 75 mg tablets of clopidogrel plus aspirin placebo or 325 mg aspirin plus clopidogrel placebo tablets", "initial supply of study drug had a sealed treatment code label attached which once opened, could not be resealed in its original form. This was retained by centre for emergency purposes". "At the close of the study, a representative sample of 3358 code‐break labels were retrieved...to verify that there were no unreported code‐break labels". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "external validation committee used"' Comment: most likely done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 42 in whole study lost to follow‐up: 22/9599(0.22%) clopidogrel group, 20/9586 (0.21%) aspirin group. Search company hired to look for patients lost to follow‐up. 4059 (21.2%) patients in whole study had study drug permanently discontinued: 21.3% (clopidogrel) and 21.1% (aspirin). |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported consistent with protocol. |
Coto 1989.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Single centre (Italy) study. Power calculation: not performed. ITT analysis: not performed. 20 day placebo washout period. Follow‐up length: six months. |
|
| Participants | Number of patients: 40. 19 (picotamide) versus 21 (placebo). Male : female ratio = 25 : 15. Age: 40 to 70 (average 63 ± 10.7). Inclusion criteria: functional stage II Fontaine, intermittent claudication for at least six months, a pain free walking distance of less than 300 metres, and an ABPI < 0.8 at rest. Exclusion criteria: congenital or acquired haemorrhagic diseases and uncontrolled hypertension. Patients with diabetes and with severe hepatic or renal impairment, or both, were also excluded. |
|
| Interventions | Intervention: picotamide 300mg tds. Control: placebo tds. |
|
| Outcomes | Cardiovascular events. Walking distance: pain free walking distance. ABPI. Adverse events: adverse events leading to cessation of therapy, GI symptoms (dyspepsia). |
|
| Notes | Placebo given to all patients during a washout and stabilisation period of 20 days. Only patients who had less than 20% variability in pain free walking distance and ABPI during this period were included. Source of funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised into two groups". Comment: method not clearly stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind fashion". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind fashion". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rates: 2/19 (10.5%) picotamide, 3/21 (14.3%) placebo group (P = 0.55, Fisher's exact test). Number of evaluable patient: 35 (five withdrawals: two in picotamide group due to gastric discomfort; three in placebo group ‐ two kept on smoking and one had gastric discomfort). |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available, and methods section in paper was brief. However, the type of outcomes was appropriate after taking into account the objectives, length of study and sample size. |
DAVID 2004.
| Methods | Randomised, double blinded trial. Multicentre (86 centres in Italy) study. Study enrolment: February 1996 to October 1998. Power calculation: 584 patients per group were required to detect an absolute reduction of mortality under picotamide of 3.5% in two years (assuming a two‐year mortality rate of 6.5% in the aspirin group), with a power of 80% and a significance level set at 0.05 (two‐sided). ITT analysis: performed. Follow‐up length: 24 months (IQR 1.9 to 2.1 years). |
|
| Participants | Number of patients: 1209. 603 (picotamide) versus 606 (aspirin). Male : female ratio = 878 : 331. Age (mean with SD): 63.8 ± 7.2 (picotamide), 64.6 ± 7.3 (aspirin). Inclusion criteria: aged between 40 and 75 years old, and with history of type II diabetes for five years or more and PAD. PAD defined as the presence of two or more of the following: 1) history of intermittent claudication lasting more than two months; 2) loss of posterior tibial pulse in the foot; 3) ABPI < 0.90 or > 1.30 in the posterior or anterior tibial artery of the foot; 4) amputation or reconstructive surgery in patients with previous history of intermittent claudication; 5) angioplasty with no persisting complication from intervention. Exclusion criteria: myocardial infarction, stroke or unstable angina in the six months prior to enrolment; severe neurological or mental deficits likely to make the patient non‐compliant; severe co‐morbidity likely to limit patient's life expectancy to less than two years; serum creatinine > 2.0 mg/dL; high risk of endo‐ocular bleeding; alanine aminotransferase or aspartate aminotransferase over three times the upper limit of normal; platelets < 100,000 per mm3; active peptic ulcer or gastro‐enteric bleeding in the six months prior to enrolment; pregnancy; severe, uncontrolled hypertension; total cholesterol level ≥ 300 mg/dL; picotamide or aspirin sensitivity. Patients scheduled for major surgery or requiring long term anti‐coagulant treatment were also excluded. |
|
| Interventions | Intervention: picotamide 600 mg bd. Control: aspirin 320 mg (every morning) plus placebo (evening). |
|
| Outcomes | All cause mortality. Cardiovascular mortality: MI or stroke related death. Cardiovascular events: fatal and non‐fatal MI or stroke. Amputation. Adverse events: adverse events leading to cessation of therapy; major bleeding (requiring hospitalisation), GI symptoms. |
|
| Notes | Source of funding: Novartis S.p.a. Number of patients available for data differ between data points. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation list was performed stratified by centre with treatment in balanced blocks of four patients within each centre, a 1:1 treatment allocation was used”. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "blinding was maintained by the use of indistinguishable active drugs and placebo tablets in separate bottles labelled for morning and evening intake". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Around 20% of patients in each group was lost to follow‐up for the secondary end‐points (mortality plus non‐fatal vascular events). Lost to follow‐up : 32/603(5.3%) picotamide, 26/606 (4.3%) aspirin (P = 0.41, Chi2 test). Treatment discontinued early: 159/603 in picotamide and 160/606 in aspirin
|
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported consistent with protocol. |
EMATAP 1993.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Multicentre (21 centres in Argentina) study. Power calculation: based on a 10% rate of outcome events in the placebo group, with an expected rate of 3.5% in the ticlopidine group, it was proposed to recruit 600 patients. This would give a 95% chance of demonstrating a significant difference in outcome between the two treatment groups at the one‐sided significance level of 5%. ITT analysis: performed. Lost to follow‐up considered as failure, for example, occurrence of an outcome event at the date of the last visit except if investigation proves that patient was event free at the end of his planned study period. Patients stratified into diabetic and non‐diabetic groups. Follow‐up length: 24 weeks. |
|
| Participants | Number of patients: 615 (out of 771 patients screened). 304 (ticlopidine) versus 311 (placebo). Male : female ratio = 521 : 94. Patients in "diabetic" stratum: 88/304 ticlopidine, 95/311 placebo Age (mean, range): 63.3 (ticlopidine); 62.5 (placebo) (range 42 to 76 years). Inclusion criteria: obstructive arterial disease of the upper part (popliteal or above) of the lower limbs for at least 12 months, confirmed by angiography (< 6 months) or doppler studies (< 3 months) and intermittent claudication with a walking distance assessed by treadmill testing (3.2 km/h, 10% slope, ambient temperature 22 ± 2 oC) of between 50 and 300 m. Exclusion criteria: < 35 or > 75 years old, disease of stage III or IV Fontaine's classification, insulin treated diabetes, vascular surgery within the past 12 months or planned for the following six months, myocardial infarction or acute stroke within the last three months, severe hypertension not adequately controlled, treatment with anti‐inflammatory or anti‐coagulant agents, contraindications to ticlopidine, aspartate aminotransferase > 50 iu/L, serum creatinine >270 μmol/L, platelets < 150 x 109 /L and granulocytes < 1.8 x 109 /L. |
|
| Interventions | Intervention: ticlopidine 250 mg bd (ratio of patients given sugar to film coated tablets 1:1). Control: placebo bd. |
|
| Outcomes | All cause mortality. Vascular mortality: stroke or MI related death. Cardiovascular events: fatal and non‐fatal MI or stroke. Walking distance: graphical data only. Need for revascularisation. Adverse events: adverse events leading to cessation of therapy, GI symptoms. |
|
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised, stratified, placebo controlled, double‐blind study", "randomisation scheme predetermined for each centre". Comment: method of sequence generation not described. Probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "each (treatment) box was clearly labelled with random treatment number", "no codes were reported as broken". Comment: treatment identity contained in "sealed envelopes" (? opaque) for emergency purposes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind trial", "placebo tablets were identical in appearance and packaging", "treatment was balanced in blocks of four tablets: ticlopidine film coated, placebo film coated, ticlopidine sugar coated, placebo sugar coated". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind study", "validated committee unaware of the nature of the treatment allocated validated outcomes and adverse events". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "a lost to follow‐up will be considered as a failure". 17/304 (5.6%) ticlopidine versus 17/311 (5.5%) placebo did not turn up for the last follow‐up visit, but: Quote: "it was possible after telephone or family contact or through their general practitioner to assert that none of these patients had experienced a critical event from their last visit". Comment: this is probably low risk due to balanced rate of not turning up, and small number. Patients were also eventually followed up. Treatment discontinued early: 42/304 (13.8%) ticlopidine group, 38/311 (12.3%) placebo group (P = 0.56, Chi2 test) due to adverse events: 20/304 (6.6%) ticlopidine, 14/311 (4.5%) placebo (P = 0.26, Chi2 test). Comment: comparable drop out rate, low risk overall. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported consistent with protocol. |
Signorini 1988.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Single centre (Italy) study. Power calculation: not performed. ITT analysis: not performed. Follow‐up length: six months |
|
| Participants | Number of patients: 52. 28 (Indobufen) versus 24 (placebo), six patients dropped out "for reasons unrelated to treatment" Male : female ratio = 39 : 13. Age: Mean 60 (range 46 to 70). Inclusion criteria: stage II Fontaine with disease of one to five years. Exclusion criteria: Occlusive thromboangiitis (Buerger), congential or acquired haemorrhagic diseases, diabetes, hyperlipoproteinaemia, smokers, angina and severe hypertension requiring continuous therapy, or both. Patients were excluded if they had concomitant diseases that would have required other associated therapies. |
|
| Interventions | Intervention: indobufen 200 mg bd. Control: placebo bd. |
|
| Outcomes | Walking distance: pain free walking distance (assessed on a treadmill at constant slope and speed (10o and 4 km/h). Adverse events: gastrointestinal symptoms. |
|
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Preset random design". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Preset random design". Comment: did not specify. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind study". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "6 patients dropped out of the trial during the first month for reasons unrelated to the treatment". Comment: did not specify attrition rate of groups. |
| Selective reporting (reporting bias) | Unclear risk | Brief methods section in paper Comment: Outcomes reported is as expected from the objectives and design of the study. |
STIMS 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled trial. Multicentre (six Swedish centres) study. Power calculation: 90% power at 5% significance levels to detect a 50% reduction of primary end points. ITT analysis: performed. Study period: November 1980 to December 1987. Stratified into presence or absence of previous leg vascular surgery. Follow‐up length: seven years (median 5.6 years). |
|
| Participants | Number of patients: 687. 346 (ticlopidine) versus 341 (placebo). Male : female ratio = 525 : 162. Age (mean with SD): 60.5 ± 6.0 (ticlopidine), 60.2 ± 6.9 (placebo). Inclusion criteria: history of intermittent claudication. Exclusion criteria: age > 70 years; pregnant and fertile women; patients receiving treatment known to affect platelet function or anticoagulant drugs; platelet count below 100 X 109 /L; patients receiving lipid lowering drugs other than clofibrate; myocardial infarction within last three months; major surgery within the last month; diabetics who were treated with insulin or who showed evidence of advanced proliferative retinopathy or renal failure; patients with chronic disease such as malignancy signs of polyneuritis of obscure origin, uncontrolled hypertension (> 190/110 mmHg), rheumatic valvular disease with atrial fibrillation, previous bleeding peptic ulcer, or a stroke which prevented them from walking to the extent that claudication could not be experienced; renal dysfunction (serum creatinine > 150 mmol/L); or liver dysfunction (aspartate aminotransferase > 2 kat/L, alanine aminotransferase > 2 μkat/L, gamma glutamyl transferase > 3 μkat/L; 1 μkat = 1 μmol of reaction product per second). |
|
| Interventions | Intervention: ticlopidine 250 mg bd. Control: placebo bd. |
|
| Outcomes | All cause mortality. Vascular mortality: stroke or MI related death. Cardiovascular events: fatal and non‐fatal MI, stroke or TIA. Need for vascular interventions and amputations. Adverse events: major bleeding and adverse events requiring cessation of therapy. |
|
| Notes | Patients who had undergone reconstructive surgery or amputation were also eligible for this study. Source of funding: Sanofi‐Winthrop. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "minimisation procedure was used to balance the placebo and ticlopidine treatment group". |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated. Comment: probably used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind placebo controlled trial", "placebo tablets were identical in appearance and packaging". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind placebo controlled trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop out rate: 5/346 (1.4%) ticlopidine; 2/341 (0.59%) placebo (P = 0.26, Chi2 test) are comparable between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported was as expected from the objectives and design of the study. |
Tonnesen 1993.
| Methods | Randomised, double blinded, placebo controlled trial. Multi‐centre (10 multinational centres ‐ Argentina, Brazil, Denmark, England, Germany, Spain and Yugoslavia) study. Power calculation: 144 evaluable patients per study arm were required to detect an expected improvement of 30% for the placebo group and 50% for the indobufen group with a power of 0.8 using a two‐tailed test at an α level of 0.05. ITT analysis: performed. Single‐blinded placebo run‐in period for one month. Follow‐up length: six months. |
|
| Participants | Number of patients: 302. 148 (indobufen) versus 154 (placebo). Male : female ratio = 216 : 100. Age: Median 62 years (range 43 to 78). Inclusion criteria: stable symptomatic, moderately severe chronic occlusive PAD of the lower extremities due to atherosclerosis. Claudication was considered stable if no significant change in the severity of symptoms had occurred in the six months prior to patient enrolment. After enrolment, single blinded placebo run in was conducted for one month, and only patients who still remain within inclusion criteria were randomised. Inclusion: intermitted claudication for at least six months prior to enrolment, ability to walk at least 50 m before complaining of pain in leg (initial claudication distance, ICD) , but no more than 300 m, as assessed on a motorised treadmill set at a slope of 8 degrees, at a speed of 3.2 km/hour. Absolute claudication distance (ACD) less than 500 m, resting ankle/arm systolic blood pressure in the arteries of the worse leg between 0.5 to 0.9. Exclusion criteria: diabetics. Use of antiplatelets, analgesic and anti‐inflammatory drugs other than paracetamol were disallowed. |
|
| Interventions | Intervention: indobufen 200 mg bd. Control: placebo bd. |
|
| Outcomes | Walking distance: ICD and ACD. Adverse events: adverse events leading to cessation of therapy, GI symptoms including dyspepsia and discomfort. |
|
| Notes | Source of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients who qualified for the double‐blind phase were randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients who qualified for the double‐blind phase were randomly allocated". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all the patients were randomly allocated to either indobufen or placebo treatment under double‐blind conditions." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blinded study". Comment: no further description. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomised patients included in data analysis‐ however there were some patients who were unaccounted for in adverse event reports. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported was as expected from the objectives and design of the study. |
ABPI: ankle brachial pressure index bd: twice a day GI: gastrointestinal ITT: intention‐to‐treat IQR: interquartile range MI: myocardial infarction NSAID: non‐steroidal anti‐inflammatory drug od: once a day SD: standard deviation tds: three times a day TIA: transient ischaemic attack WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrahamsen 1974 | Cross‐over study. Patient given antiplatelet for two weeks only. No primary or secondary outcomes reported. |
| Adriaensen 1976 | Used suloctidil and dihydroergotoxine as drugs of investigation. |
| Ahn 1992 | Compared antiplatelet agent against surgery. |
| Allegra 1993 | Compared antiplatelet agent against low dose heparin. See Allegra 1994. |
| Allegra 1994 | Compared antiplatelet agent against low dose heparin. |
| Andreozzi 1993 | Compared antiplatelet against low dose heparin. |
| Baumgartner 1992 | No suitable results for primary and secondary outcomes. |
| Belcaro 1991 | No mention of randomisation process. No placebo used. Control group was made up of patients who were unable to follow any kind of treatment (either because they refused it or because they had important gastrointestinal symptoms). |
| Bhatt 2000 | Part of CAPRIE study. Outcome for PAD patients specifically not reported. |
| Boneu 1996 | Trial to assess dose of clopidogrel only. No primary or secondary outcomes reported. |
| Brass 2006 | Used vasodilator (NM‐702) as drug of investigation. |
| Canonico 1991 | Cross‐over trial. |
| Cassar 2005 | Recruited patients undergoing angioplasty. |
| Cassar 2005a | Recruited patients undergoing angioplasty, see Cassar 2005. |
| Cassar 2006 | Ex‐vivo measure of platelet activation. |
| Castano 1999 | Used policosanol as drug of investigation. |
| Castano 2001 | Used policosanol as drug of investigation. |
| Castano 2003 | Used policosanol as drug of investigation. |
| Castano 2004 | Used policosanol as drug of investigation. |
| Catalano 1984 | Unclear patient grouping. |
| Cesarone 1994 | Used defibrotide as comparator. |
| CHARISMA 2009 | Included asymptomatic PAD participants. |
| Ciocon 1997 | Used pentoxifylline as comparator. |
| CLIPS 2007 | Included asymptomatic PAD participants. |
| Creutzig 1994 | Recruited patients undergoing angioplasty. |
| Davi 1985 | Cross‐over trial. Patients received treatment for 60 days only. |
| Destors 1985 | Proposal for trial. |
| Duda 2001 | Used thrombolysis agent as comparator. |
| Ehresmann 1977 | Patients had undergone recent revascularisation. |
| Eikelboom 2005 | Cross‐over trial with no primary or secondary outcomes reported. |
| Elam 1998 | Used cilostazol as drug of investigation. |
| Fabris 1992 | Included patients with Fontaine stage III. |
| Fiotti 2003 | Non‐randomised study. |
| Fowkes 2010 | Included asymptomatic PAD participants. |
| Giansante 1990 | Unusual dosing ‐ patient received antiplatelet therapy every third day. |
| Gillot 1976 | Used suloctidil as drug of investigation. |
| Gregoratti 1982 | Used suloctidil as drug of investigation. |
| Gresele 2000 | Used clomicromene, an antithrombotic as part of comparator. |
| Guan 2003 | Used beraprost as drug of investigation. |
| Harker 1999 | Part of CAPRIE study. Outcomes for PAD patients specifically, was not reported. |
| Hawker 1984 | No primary or secondary outcomes reported. |
| Hess 1985 | No primary or secondary outcomes reported. |
| Hevia 1992 | Used nifedipine as comparator. |
| Hiatt 2002 | Used urokinase as drug of investigation. |
| Holm 1984 | Used suloctidil as drug of investigation. |
| Hsieh 2009 | Used cilostazol as drug of investigation. Patients also received concomitant antiplatelet. |
| Jagroop 2004 | Patients only received 16 days of treatment. |
| Jones 1982 | Used suloctidil as drug of investigation. |
| Kakkar 1981 | Used suloctidil as drug of investigation. |
| Katsumura 1982 | Included patients with ischaemic ulcers. |
| Labs 1999 | Used beraprost as drug of investigation at varying doses. |
| Landini 1989 | Patients followed up for one month only. |
| Leo 2007 | Treatment duration was only two months. |
| Libretti 1986 | No primary or secondary outcomes reported. |
| Libretti 1986a | No primary or secondary outcomes reported. |
| Lievre 1996 | Used beraprost as main drug of investigation. |
| Lievre 2000 | Used beraprost as main drug of investigation. |
| Mangiafico 2000 | Four week treatment duration only. Used prostaglandin as drug of investigation. |
| Mannarino 1991 | Compared antiplatelet to exercise. |
| Mannucci 1987 | No comparator used. |
| Mantero 1983 | Used suloctidil as drug of investigation. |
| Marelli 1990 | Used defibrotide as drug of investigation. |
| Marrapodi 1994 | Used defibrotide as drug of investigation. |
| Miyazaki 2007 | Used sarpogrelate as drug of investigation. |
| Mohler 2003 | Used beraprost as drug of investigation. |
| Neirotti 1994 | No primary or secondary outcomes reported. |
| Nenci 1979 | Cross‐over trial. |
| Nenci 1982 | Cross‐over trial. |
| Norgren 2006 | Used sarpogrelate as drug of investigation. |
| Novo 1996 | Cross‐over trial. Used captopril as comparator. |
| O'Donnell 2008 | Used cilostazol as drug of investigation. |
| O'Donnell 2009 | Used cilostazol as drug of investigation. |
| Okadome‐Kenchiro 1992 | Used vasodilator as drug of investigation. |
| Panchenko 1997 | Used pentoxifylline as drug of investigation. |
| Pasqualini 2002 | Only assessed walking distance after day 2 to 5 of treatment. |
| Pollastri 1991 | Used trapidil as drug of investigation. |
| POPADAD 2008 | Included participants with asymptomatic PAD. |
| Randi 1985 | Not RCT. Included stage III and IV Fontaine. |
| Randi 1991 | No primary or secondary outcomes reported. |
| Ranke 1992 | Recruited patients following angioplasty. |
| Ranke 1993 | Recruited patients following angioplasty. |
| Ranke 1994 | Recruited patients following angioplasty. |
| Regenthal 1991 | Used trapidil and pentoxifylline as drugs of investigation. |
| Rossini 1998 | Used heparan sulphate as comparator. |
| Roztocil 1989 | Compared antiplatelet against hydroxyethylrutoside. |
| Rudofsky 1987 | No primary or secondary outcomes reported |
| Schoop 1987 | No primary or secondary outcomes reported. |
| Schweizer 2003 | Recruited patients with acute arterial thrombosis. |
| Singer 2006 | Trial proposal only. |
| Smith 1981 | Not RCT. |
| Stiegler 1984 | Used angiography to determine outcome. |
| Tepe 2007 | Trial proposal only. |
| Topol 2000 | Included patients with coronary heart disease and cerebrovascular disease. Data for PAD patients could not be obtained. |
| Warfarin 2007 | Used warfarin as comparator. |
| Wilhite 2003 | Cross‐over trial. |
Differences between protocol and review
The interpretation of the types of participants, the interventions and outcomes as described in protocol of this review (Robless 2003) were in some instances unclear and the reviewers have therefore provided clarification by providing definitions of patients with PAD, antiplatelet agents and cardiovascular events and adverse events.
The initial published protocol proposed to perform analysis on quality of life (QoL) and cost effectiveness. However, none of the studies included in this review reported these data, and it was not possible to perform these analyses. The types of studies included in the review were narrowed down compared with the protocol by excluding quasi‐randomised and cross‐over trials to reduce the risk of biases from non‐random sequence generation and difficulty in attributing the events to the intervention in question, respectively. The required length of the intervention was increased from 4 weeks to 3 months in order to reduce the potential of bias from under‐reporting from a short period of treatment, and also to allow real assessment of antiplatelet agents and their longer term impact on mortality.
The assessment of the methodological quality of the included studies was changed to the updated Cochrane Collaboration's recommended 'Risk of bias' tool (Higgins 2011a).
Contributions of authors
Peng Wong (PW): selected trials, extracted data, assessed risk of bias, analysed and interpreted the data, and drafted manuscript. Lee‐Yee Chong (LYC): selected trials, extracted data, assessed risk of bias,analysed and interpreted the data, and drafted manuscript. Dimitri Mikhailidis (DM): provided advice on antiplatelets and appraised the manuscript. Peter Robless (PR): interpreted the data, appraised the manuscript. Gerard Stansby (GS): resolved disagreements on inclusion, interpreted the data, drafted and appraised the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
Declarations of interest
DM: "I have attended meetings and advisory boards and given some lectures sponsored by Merck, Sharp and Dohme (MSD) and Genzyme. I am editor‐in‐chief of several journals. Hence some royalties are paid to me (Bentham publications, SAGE publications and Informa publications)."
New
References
References to studies included in this review
ADEP 1993 {published data only}
- Balsano F, Violi F, and the ADEP Group. Effect of Picotamide on the Clinical Progression of Peripheral Vascular Disease. A Double‐Blind Placebo‐Controlled Study. Circulation 1993;87(5):1563‐9. [DOI] [PubMed] [Google Scholar]
- Violi F, Criqui M, Longoni A, Castiglioni C. Relation between risk factors and cardiovascular complications in patients with peripheral vascular disease. Results from the A.D.E.P. study. Atherosclerosis 1996;120(1‐2):25‐35. [DOI] [PubMed] [Google Scholar]
Arcan 1988 {published data only}
- Arcan JC, Blanchard J, Boissel JP, Destors JM, Panak E. Multicenter double‐blind study of ticlopidine in the treatment of intermittent claudication and the prevention of complications. Angiology 1988;39(9):802‐11. [DOI] [PubMed] [Google Scholar]
Aukland 1982 {published data only}
- Aukland A, Hurlow RA, George AJ, Stuart J. Platelet inhibition with Ticlopidine in atherosclerotic intermittent claudication. Journal of Clinical Pathology 1982;35(7):740‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Auteri 1995 {published data only}
- Auteri A, Angaroni A, Borgatti E, Catalano M, Vizzi GB, Forconi S, et al. Triflusal in the treatment of patients with chronic peripheral arteriopathy: multicentre double‐blind study vs placebo. International Journal of Clinical and Pharmacology Research 1995;15(2):57‐63. [PubMed] [Google Scholar]
Balsano 1989 {published data only}
- Balsano F, Coccheri S, Libretti A, Nenci GG, Catalano M, Fortunato G, et al. Ticlopidine in the treatment of intermittent claudication: a 21‐month double‐blind trial. Journal of Laboratory and Clinical Medicine 1989;114(1):84‐91. [PubMed] [Google Scholar]
CAPRIE 1996 {published data only}
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348(9038):1329‐39. [DOI] [PubMed] [Google Scholar]
Coto 1989 {published data only}
- Coto V, Cocozza M, Oliviero U, Lucariello A, Picano T, Coto F, et al. Clinical efficacy of picotamide in long‐term treatment of intermittent claudication. Angiology 1989;40(10):880‐5. [DOI] [PubMed] [Google Scholar]
DAVID 2004 {published data only}
- Neri Serneri GG, Coccheri S, Marubini E, Violi F and for the Committees and the Investigators of the Drug Evaluation in Atherosclerotic Vascular Disease in Diabetics (DAVID) Study Group. Picotamide, a combined inhibitor of thromboxane A2 synthase and receptor, reduces 2‐year mortality in diabetics with peripheral arterial disease: the DAVID study. European Heart Journal 2004;25(20):1845‐52. [DOI] [PubMed] [Google Scholar]
EMATAP 1993 {published data only}
- Blanchard J, Carreras LO, Kindermans M. Results of EMATAP: a double‐blind placebo‐controlled multicentre trial of ticlopidine in patients with peripheral arterial disease. Nouvelle Revue francaise d'hematologie 1994;35(6):523‐8. [PubMed] [Google Scholar]
- Blanchard JF, Carreras LO, The EMATAP Group. A double‐blind, placebo‐controlled multicentre trial of ticlopidine in patients with peripheral arterial disease in Argentina. Design, organization and general characteristics of patients at entry. Nouvelle Revue Francaise d'Hematologie 1992;34(2):149‐53. [PubMed] [Google Scholar]
Signorini 1988 {published data only}
- Signorini GP, Salmistraro G, Maraglino G. Efficacy of indobufen in the treatment of intermittent claudication. Angiology 1988;39(8):742‐6. [DOI] [PubMed] [Google Scholar]
STIMS 1990 {published data only}
- Bergqvist D, Almgren B, Dickinson JP. Reduction of requirement for leg vascular surgery during long‐term treatment of claudicant patients with ticlopidine: results from the Swedish Ticlopidine Multicentre Study (STIMS). European Journal of Vascular & Endovascular Surgery 1995;10(1):69‐76. [DOI] [PubMed] [Google Scholar]
- Fagher B. Long‐term effects of ticlopidine on lower limb blood flow, ankle/brachial index and symptoms in peripheral arterioschlerosis. A double‐blind study. The STIMS Group in Lund. Swedish Ticlopidine Multicenter Study. Angiology 1994;45(9):777‐88. [DOI] [PubMed] [Google Scholar]
- Janzon L. The STIMS trial: the ticlopidine experience and its clinical applications. Swedish Ticlopidine Multicenter Study. Vascular Medicine 1996;1(2):141‐3. [DOI] [PubMed] [Google Scholar]
- Janzon L, Bergqvist D, Boberg J, Eriksson I, Lindgarde F, Persson G, et al. Prevention of myocardial infarction and stroke in patients with intermittent claudication; effect of ticlopidine. Results from STIMS, the Swedish Ticlopidine Multicentre Study. Journal of Internal Medicine 1990;227(5):301‐8. [DOI] [PubMed] [Google Scholar]
Tonnesen 1993 {published data only}
- Tonnesen KH, Albuquerque P, Baitsch G, Alonso AG, Ibanez F, Kester RC, et al. Double‐blind, controlled, multicenter study of indobufen versus placebo in patients with intermittent claudication. International Angiology 1993;12(4):371‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Abrahamsen 1974 {published data only}
- Abrahamsen AF, Eika C, Godal HC, Lorentsen E. Effect of acetylsalicylic acid and dipyridamole on platelet surivival and aggregation in patients with atherosclerosis obliterans. Scandinavian Journal of Haematology 1974;13(4):241‐5. [DOI] [PubMed] [Google Scholar]
Adriaensen 1976 {published data only}
- Adriaensen H. Medical treatment of intermittent claudication: a comparative double‐blind study of suloctidil, dihydroergotoxine and placebo. Current Medical Research and Opinion 1976;4(6):395‐401. [DOI] [PubMed] [Google Scholar]
Ahn 1992 {published data only}
- Ahn S, Rutherford RB. A multicenter prospective randomized trial to determine the optimal treatment of patients with claudication and isolated superficial femoral artery occlusive disease: conservative versus endovascular versus surgical therapy. Journal of Vascular Surgery 1992;15(5):889‐91. [DOI] [PubMed] [Google Scholar]
Allegra 1993 {published data only}
- Allegra C, Carlizza A, Sardina M. Long‐term effects of low‐dose calcium‐heparin versus ASA in patients with peripheral arterial occlusive disease at IIb Leriche Fontaine stage. Thrombosis & Haemostasis 1993; Vol. 69, issue 6:653‐Abstract No 401.
Allegra 1994 {published data only}
- Allegra C, Pollari G, Carioti B, Sardina M. Thrombin and platelet inhibition with low‐dose calcium‐heparin in comparison with ASA in patients with peripheral arterial occlusive disease at Leriche‐Fontaine IIb class. International Journal of Clinical Pharmacology & Therapeutics 1994;32(12):646‐51. [PubMed] [Google Scholar]
Andreozzi 1993 {published data only}
- Andreozzi GM, Signorelli SS, Cacciaguerra G, Pino L, Martini R, Monaco S, et al. Three‐month therapy with calcium‐heparin in comparison with ticlopidine in patients with peripheral arterial occlusive disease at Leriche‐Fontaine IIb class. Angiology 1993;44(4):307‐13. [DOI] [PubMed] [Google Scholar]
Baumgartner 1992 {published data only}
- Baumgartner I, Schalch I, Bollinger A. [Double blind study of the effects of dipyridamol in patients with intermittent claudication]. Vasa 1992;21(4):387‐91. [PubMed] [Google Scholar]
Belcaro 1991 {published data only}
- Belcaro G, Simone P. Long‐term evaluation of indobufen in peripheral vascular disease. Angiology 1991;42(1):8‐14. [DOI] [PubMed] [Google Scholar]
Bhatt 2000 {published data only}
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. American Heart Journal 2000;140(1):67‐73. [DOI] [PubMed] [Google Scholar]
Boneu 1996 {published data only}
- Boneu B, Destelle G. Platelet anti‐aggregating activity and tolerance of clopidogrel in atherosclerotic patients. Thrombosis & Haemostasis 1996;76(6):939‐43. [PubMed] [Google Scholar]
Brass 2006 {published data only}
- Brass EP, Anthony R, Cobb FR, Koda I, Jiao J, Hiatt WR. The novel phosphodiesterase inhibitor NM‐702 improves claudication‐limited exercise performance in patients with peripheral arterial disease. Journal of the American College of Cardiology 2006;48(12):2539‐45. [DOI] [PubMed] [Google Scholar]
Canonico 1991 {published data only}
- Canonico V, Ammaturo V, Guarini P, Tedeschi C, Nunziata G, Nappi A, et al. The clinico‐instrumental evaluation of the efficacy of picotamide in treating chronic obstructive arteriopathies of the lower extremities [Valutazione clinico‐strumentale della efficacia della picotamide nel trattamento delle arteriopatie croniche ostruttive degli arti inferiori]. Minerva Cardioangiologica 1991;39(3):75‐80. [PubMed] [Google Scholar]
Cassar 2005 {published data only}
- Cassar K, Ford I, Greaves M, Bachoo P, Brittenden J. Randomized clinical trial of the antiplatelet effects of aspirin‐clopidogrel combination versus aspirin alone after lower limb angioplasty. British Journal of Surgery 2005;92(2):159‐65. [DOI] [PubMed] [Google Scholar]
Cassar 2005a {published data only}
- Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Clopidogrel has no effect on D‐dimer and thrombin‐antithrombin III levels in patients with peripheral arterial disease undergoing peripheral percutaneous transluminal angioplasty. Journal of Vascular Surgery 2005;42(2):252‐8. [DOI] [PubMed] [Google Scholar]
Cassar 2006 {published data only}
- Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Variability in responsiveness to clopidogrel in patients with intermittent claudication. European Journal of Vascular & Endovascular Surgery 2006;32(1):71‐5. [DOI] [PubMed] [Google Scholar]
Castano 1999 {published data only}
- Castano G, Mas R, Roca J, Fernandez L, Illnait J, Fernandez JC, et al. A double‐blind, placebo‐controlled study of the effects of policosanol in patients with intermittent claudication. Angiology 1999;50(2):123‐30. [DOI] [PubMed] [Google Scholar]
Castano 2001 {published data only}
- Castano G, Mas Ferreiro R, Fernandez L, Gamez R, Illnait J, Fernandez C. A long‐term study of policosanol in the treatment of intermittent claudication. Angiology 2001;52(2):115‐25. [DOI] [PubMed] [Google Scholar]
Castano 2003 {published data only}
- Castano G, Mas R, Fernandez L, Gamez R, Illnait J. Effects of policosanol and lovastatin in patients with intermittent claudication: a double‐blind comparative pilot study. Angiology 2003;54(1):25‐38. [DOI] [PubMed] [Google Scholar]
Castano 2004 {published data only}
- Castano G, Mas R, Gamez R, Fernandez L, Illnait J. Effects of policosanol and ticlopidine in patients with intermittent claudication: a double‐blinded pilot comparative study. Angiology 2004;55(4):361‐71. [DOI] [PubMed] [Google Scholar]
Catalano 1984 {published data only}
- Catalano M, Libretti A. Dipyridamole combined with acetylsalicylic acid versus acetylsalicylic acid alone in the treatment of peripheral vascular disease. International Angiology 1984;3(3):321‐2. [Google Scholar]
Cesarone 1994 {published data only}
- Cesarone MR, Laurora G, Sanctis MT, Incandela L, Belcaro G. Vasospasm in patients with intermittent claudication: Effects of defibrotide and acetylsalicylic acid. Advances in Therapy 1994;11(2):62‐9. [Google Scholar]
CHARISMA 2009 {published data only}
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. Journal of the American College of Cardiology 2007;49(19):1982‐8. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. CHARISMA Investigators. A global view of atherothrombosis: baseline characteristics in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. [Erratum appears in Am Heart J. 2006 Jan;151(1):247]. American Heart Journal 2005;150(3):401. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Topol EJ, Clopidogrel for High Atherothrombotic Risk, Ischemic Stabilization. Management, and Avoidance Executive Committee. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high‐risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. American Heart Journal 2004;148(2):263‐8. [DOI] [PubMed] [Google Scholar]
- Cacoub PP, Bhatt DL, Steg PG, Topol EJ, Creager MA. CHARISMA Investigators. Patients with peripheral arterial disease in the CHARISMA trial. European Heart Journal 2009;30(2):192‐201. [DOI] [PubMed] [Google Scholar]
Ciocon 1997 {published data only}
- Ciocon JO, Galindo‐Ciocon D, Galindo DJ. A comparison between aspirin and pentoxifylline in relieving claudication due to peripheral vascular disease in the elderly. Angiology 1997;48(3):237‐40. [DOI] [PubMed] [Google Scholar]
CLIPS 2007 {published data only}
- Catalano M, Born, G, Peto R. Critical Leg Ischaemia Prevention Study (CLIPS) Group, et al. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double‐blind trial. Journal of Internal Medicine 2007;261(3):276‐84. [DOI] [PubMed] [Google Scholar]
Creutzig 1994 {published data only}
Davi 1985 {published data only}
- Davi G, Pinto A, Francavilla G, Paterna S, Campisi D, Strano A. Inhibition of platelet functon by ticlopidine in arteriosclerosis obliterans of the lower limbs. Thrombosis Research 1985;40(2):275‐81. [DOI] [PubMed] [Google Scholar]
Destors 1985 {published data only}
- Destors JM, Arcan JC. Evaluation of oral drugs for intermittent claudication of the legs in phase III clinical trials. Options selected for the ACT study. Therapie 1985;40(6):451‐8. [PubMed] [Google Scholar]
Duda 2001 {published data only}
- Duda SH, Tepe G, Luz O, Ouriel K, Dietz K, Hahn U, et al. Peripheral artery occlusion: treatment with abciximab plus urokinase versus with urokinase alone‐a randomized pilot trial (the PROMPT Study). Platelet Receptor Antibodies in Order to Manage Peripheral Artery Thrombosis. Radiology 2001;221(3):689‐96. [DOI] [PubMed] [Google Scholar]
Ehresmann 1977 {published data only}
- Ehresmann U, Alemany J, Loew D. [Use of acetylsalicylic acid in the prevention of re‐occlusion following revascularization interventions. Results of a double‐ blind long term study]. Die Medizinische Welt 1977;28(26):1157‐62. [PubMed] [Google Scholar]
Eikelboom 2005 {published data only}
- Eikelboom JW, Hankey GJ, Thom J, Claxton A, Yi Q, Gilmore G, et al. Enhanced antiplatelet effect of clopidogrel in patients whose platelets are least inhibited by aspirin: a randomized crossover trial. Journal of Thrombosis & Haemostasis 2005;3(12):2649‐55. [DOI] [PubMed] [Google Scholar]
Elam 1998 {published data only}
- Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, et al. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arteriosclerosis, Thrombosis and Vascular Biology 1998;18(12):1942‐7. [DOI] [PubMed] [Google Scholar]
Fabris 1992 {published data only}
- Fabris F, Steffan A, Randi ML, Avruscio GP, Cordiano I, Girolami A. Indobufen versus dipyridamole plus aspirin in the treatment of patients with peripheral atherosclerotic disease. Journal of Medicine 1992;23(2):81‐92. [PubMed] [Google Scholar]
Fiotti 2003 {published data only}
- Fiotti N, Altamura N, Cappelli C, Schillan M, Guarnieri G, Giansante C. Long term prognosis in patients with peripheral arterial disease treated with antiplatelet agents. European Journal of Vascular & Endovascular Surgery 2003;26(4):374‐80. [DOI] [PubMed] [Google Scholar]
Fowkes 2010 {published data only}
- Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010;303(9):841‐8. [DOI] [PubMed] [Google Scholar]
Giansante 1990 {published data only}
- Giansante C, Calabrese S, Fisicaro M, Fiotti N, Mitri E. Treatment of intermittent claudication with antiplatelet agents. The Journal of International Medical Research 1990;18(5):400‐7. [DOI] [PubMed] [Google Scholar]
Gillot 1976 {published data only}
- Gillot P. A double‐blind study comparing suloctidil and placebo in chronic peripheral arteriopathy. Current Therapeutic Research 1976;20(5):637‐44. [PubMed] [Google Scholar]
Gregoratti 1982 {published data only}
- Gregoratti L, Redaelli G, Limido P, Ghiringhelli L. Clinical and instrumental evaluation of patients with chronic peripheral arterial occlusive disease after prolonged administration of suloctidil. La Clinica Terapeutica 1982;102(6):607‐19. [PubMed] [Google Scholar]
Gresele 2000 {published data only}
- Gresele P, Migliacci R, Sante G, Nenci GG, CRAMPS Investigator Group. Effect of cloricromene on intermittent claudication. A randomized, double‐blind, placebo‐controlled trial in patients treated with aspirin: effect on claudication distance and quality of life. Vascular Medicine 2000;5(2):83‐9. [DOI] [PubMed] [Google Scholar]
Guan 2003 {published data only}
- Guan H, Wang Y, Zhang B, Ye W, Fu W, Liang W, et al. Comparison of beraprost and ticlopidine in Chinese patients with chronic peripheral arterial occlusion: a multicenter, single‐blind, randomized, controlled study. Current Therapeutic Research, Clinical and Experimental 2003;64(7):488‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harker 1999 {published data only}
- Harker LA, Boissel JP, Pilgrim AJ, Gent M. Comparative safety and tolerability of clopidogrel and aspirin: results from CAPRIE. CAPRIE Steering Committee and Investigators. Clopidogrel versus aspirin in patients at risk of ischaemic events. Drug Safety 1999;21(4):325‐35. [DOI] [PubMed] [Google Scholar]
Hawker 1984 {published data only}
- Hawker RJ, Aukland A. Platelet survival, atherosclerotic intermittent claudication and ticlopidine. Atherosclerosis 1984;50(2):147‐58. [DOI] [PubMed] [Google Scholar]
Hess 1985 {published data only}
- Hess H, Mietaschik A, Deichsel G. Drug‐induced inhibition of platelet function delays progression of peripheral occlusive arterial disease. A prospective double‐blind arteriographically controlled trial. Lancet 1985;1(8426):415‐9. [DOI] [PubMed] [Google Scholar]
Hevia 1992 {published data only}
- Hevia Alonso A, Gago Angelino J, Lopez‐Valpuesta FJ, Serrano Molina JS, Fernandez‐Sanz S. The effects of ticlopidine and nifedipine on platelet aggregation in patients with obliterant artheriopathy of the lower limbs. Revista Clinica Espanola 1992;190(6):295‐8. [PubMed] [Google Scholar]
Hiatt 2002 {published data only}
- Hiatt WR. Abciximab added to urokinase increased amputation‐free survival in peripheral arterial occlusion of the legs. ACP Journal Club 2002;137(1):12. [PubMed] [Google Scholar]
Holm 1984 {published data only}
- Holm J, Lindblad L, Schersten T, Suurkula M. Intermittent claudication: Suloctidil vs placebo treatment. Vasa 1984;13(2):175‐8. [PubMed] [Google Scholar]
Hsieh 2009 {published data only}
- Hsieh CJ, Wang PW. Effect of cilostazol treatment on adiponectin and soluble CD40 ligand levels in diabetic patients with peripheral arterial occlusion disease. Circulation Journal 2009;73(5):948‐54. [DOI] [PubMed] [Google Scholar]
Jagroop 2004 {published data only}
- Jagroop IA, Matsagas MI, Geroulakos G, Mikhailidis DP. The effect of clopidogrel, aspirin and both antiplatelet drugs on platelet function in patients with peripheral arterial disease. Platelets 2004;15(2):117‐25. [DOI] [PubMed] [Google Scholar]
Jones 1982 {published data only}
- Jones NAG, Haas HA, Zahavi J, Kakkar VV. A double‐blind trial of suloctidil versus placebo in intermittent claudication. British Journal of Surgery 1982;69(1):38‐40. [DOI] [PubMed] [Google Scholar]
Kakkar 1981 {published data only}
- Kakkar VV, Jones NAG, Haas J, Zahavi J. A double blind trial ‐ suloctidil versus placebo in intermittent claudication. Thrombosis & Haemostasis 1981; Vol. 46, issue 1:92.
Katsumura 1982 {published data only}
- Katsumura T, Mishima Y, Kamiya K, Sakaguchi S, Tanabe T, Sakuma A. Therapeutic effect of ticlopidine, a new inhibitor of platelet aggregation, on chronic arterial occlusive diseases, a double‐blind study versus placebo. Angiology 1982;33(6):357‐67. [DOI] [PubMed] [Google Scholar]
Labs 1999 {published data only}
- Labs KH, Nehler MR, Roessner M, Jaeger KA, Hiatt WR. Reliability of treadmill testing in peripheral arterial disease: a comparison of a constant load with a graded load treadmill protocol. Vascular Medicine 1999;4(4):239‐46. [DOI] [PubMed] [Google Scholar]
Landini 1989 {published data only}
- Landini G, Poggiali C, Calacoci S, Marino N, Rosselli A. Platelet aggregation inhibitory therapy in chronic arterial occlusive disease of the lower limbs: results of short‐term clinico‐instrumental study with indobufen. Recenti Progressi in Medicina 1989;80(4):204‐7. [PubMed] [Google Scholar]
Leo 2007 {published data only}
- Leo W, Westrych R, Bissinger A, Okraszewski J, Baj Z. [Effect of the acetylosalicyd acid (ASA) and ticlopidine therapy on clinical condition and parameters of blood platelets in patients with peripheral arterial occlusive disease (PAOD)]. Polski Merkuriusz Lekarski 2007;23(137):335‐9. [PubMed] [Google Scholar]
Libretti 1986 {published data only}
- Libretti A, Catalano M. Treatment of claudication with dipyridamole and aspirin. International Journal of Clinical and Pharmacology Research 1986;6(1):59‐60. [PubMed] [Google Scholar]
Libretti 1986a {published data only}
- Libretti A, Catalano M. Treatment of claudication with dipyridamole and aspirin. Monographs on Atherosclerosis 1986;14:207‐9. [PubMed] [Google Scholar]
Lievre 1996 {published data only}
- Lievre M, Azoulay S, Lion L, Morand S, Girre JP, Boissel JP. A dose‐effect study of beraprost sodium in intermittent claudication. Journal of Cardiovascular Pharmacology 1996;27(6):788‐93. [DOI] [PubMed] [Google Scholar]
Lievre 2000 {published data only}
- Lievre M, Morand S, Besse B, Fiessinger JN, Boissel JP. Oral Beraprost sodium, a prostaglandin I(2) analogue, for intermittent claudication: a double‐blind, randomized, multicenter controlled trial. Circulation 2000;102(4):426‐31. [DOI] [PubMed] [Google Scholar]
Mangiafico 2000 {published data only}
- Mangiafico RA, Messina R, Attina T, Dell'Arte S, Giuliano L, Malatino LS. Impact of a 4‐week treatment with prostaglandin E1 on health‐related quality of life of patients with intermittent claudication. Angiology 2000;51(6):441‐9. [DOI] [PubMed] [Google Scholar]
Mannarino 1991 {published data only}
- Mannarino E, Pasqualini L, Innocente S, Scricciolo V, Rignanese A, Ciuffetti G. Physical training and antiplatelet treatment in stage II peripheral arterial occlusive disease: alone or combined?. Angiology 1991;42(7):513‐21. [DOI] [PubMed] [Google Scholar]
Mannucci 1987 {published data only}
- Mannucci L, Maderna P, Colli S, Lavezzari M, Sirtori CR, Tremoli E. Indobufen is a potent inhibitor of whole blood aggregation in patients with a high atherosclerotic risk. Thrombosis Research 1987;48(4):417‐26. [DOI] [PubMed] [Google Scholar]
Mantero 1983 {published data only}
- Mantero M, Bondioli A, Catenazzo G. Suloctidil: a controlled clinical and instrumental study on patients with claudicatio intermittens. Gazzetta Medica Italiana 1983;142(10):475‐8. [Google Scholar]
Marelli 1990 {published data only}
- Marelli C, Belcaro G, Girardello R. Defibrotide in patients with intermittent claudication. Improvement in blood flow, fibrinolytic activity and microcirculation after six months of treatment. Current Therapeutic Research, Clinical and Experimental 1990;47(3):459‐65. [Google Scholar]
Marrapodi 1994 {published data only}
- Marrapodi E, Leanza D, Giordano S, Nazzari M, Corsi C. Effects of defibrotide on physical performance and hemorheologic picture in patients with peripheral arteriopathy. Clinical Trials and Meta‐Analysis 1994;29(1):21‐30. [PubMed] [Google Scholar]
Miyazaki 2007 {published data only}
- Miyazaki M, Higashi Y, Goto C, Chayama K, Yoshizumi M, Sanada H, et al. Sarpogrelate hydrochloride, a selective 5‐HT2A antagonist, improves vascular function in patients with peripheral arterial disease. Journal of Cardiovascular Pharmacology 2007;49(4):221‐7. [DOI] [PubMed] [Google Scholar]
Mohler 2003 {published data only}
- Mohler ER III, Hiatt WR, Olin JW, Wade M, Jeffs R, Hirsch AT. Treatment of intermittent claudication with beraprost sodium, an orally active prostaglandin I2 analogue: a double‐blinded, randomized, controlled trial. Journal of the American College of Cardiology 2003;41(10):1679‐86. [DOI] [PubMed] [Google Scholar]
Neirotti 1994 {published data only}
- Neirotti M, Molaschi M, Ponzetto M, Macchione C, Poli L, Bonino F, et al. Hemodynamic, hemorheologic, and hemocoagulative changes after treatment with picotamide in patients affected by peripheral arterial disease (PAD) of the lower limbs. Angiology 1994;45(2):137‐41. [DOI] [PubMed] [Google Scholar]
Nenci 1979 {published data only}
- Nenci GG, Agnelli G, Berrettini M, Parise P, Ballatori E. Inhibition of spontaneous platelet aggregation by sulfinpyrazone. Thrombosis & Haemostasis 1979;42(2):621‐5. [PubMed] [Google Scholar]
Nenci 1982 {published data only}
- Nenci GG, Berrettini M, Iadevaia V, Parise P, Ballatori E. Inhibition of spontaneous platelet aggregation and adhesion by indobufen (K 3920). A randomized, double‐blind crossover study on platelet, coagulation and fibrinolysis function tests. Pharmatherapeutica 1982;3(3):188‐94. [PubMed] [Google Scholar]
Norgren 2006 {published data only}
- Norgren L, Jawien A, Matyas L, Riegerd H, Arita K, European MCI‐9042 Study Group. Sarpogrelate, a 5‐HT2a receptor antagonist in intermittent claudication. A Phase II European study. Vascular Medicine 2006;11(2):75‐83. [DOI] [PubMed] [Google Scholar]
Novo 1996 {published data only}
- Novo S, Abrignani MG, Pavone G, Zamueli M, Pernice C, Geraci AM, et al. Effects of captopril and ticlopidine, alone or in combination, in hypertensive patients with intermittent claudication. International Angiology 1996;15(2):169‐74. [PubMed] [Google Scholar]
O'Donnell 2008 {published data only}
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The effects of cilostazol on peripheral neuropathy in diabetic patients with peripheral arterial disease. Angiology 2009;59(6):695‐704. [DOI] [PubMed] [Google Scholar]
O'Donnell 2009 {published data only}
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The vascular and biochemical effects of cilostazol in diabetic patients with peripheral arterial disease. Vascular and Endovascular Surgery 2009;43(2):132‐43. [DOI] [PubMed] [Google Scholar]
Okadome‐Kenchiro 1992 {published data only}
- Okadome‐Kenchiro, et al. Efficacy of lipo PGE1 in combination with an oral anti‐platelet agent in chronic arterial obstruction: A multicenter comparative study. Rinsho to Kenkyu 1992;69:3655‐62. [Google Scholar]
Panchenko 1997 {published data only}
- Panchenko E, Eshkeeva A, Dobrovolsky A, Titaeva E, Podinovskaya Y, Hussain KM, et al. Effects of indobufen and pentoxifylline on walking capacity and hemostasis in patients with intermittent claudication: results of six months treatment. Angiology 1997;48(3):247‐54. [DOI] [PubMed] [Google Scholar]
Pasqualini 2002 {published data only}
- Pasqualini L, Pirro M, Lombardini R, Ciuffetti G, Dragani P, Mannarino E. A human model of platelet‐leucocyte adhesive interactions during controlled ischaemia in patients with peripheral vascular disease. Journal of Clinical Pathology 2002;55(12):946‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollastri 1991 {published data only}
- Pollastri M, Saccocci M, Corsi C, Monici Preti PA, Cantini L. Controlled study on the efficacy and tolerability of trapidil in the treatment of intermittent claudication of the lower extremities. One‐year review. Archivio di Medicina Interna 1991;43(4):197‐207. [Google Scholar]
POPADAD 2008 {published data only}
- Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. British Medical Journal 2008;337:a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Randi 1985 {published data only}
- Randi ML, Fabris F, Crociani ME, Battocchio F, Girolami A. Effects of ticlopidine on blood fibrinogen and blood viscosity in peripheral atherosclerotic disease. Arzneimittel‐Forschung 1985;35(12):1847‐9. [PubMed] [Google Scholar]
Randi 1991 {published data only}
- Randi ML, Mares M, Fabris F, Tison T, Barbone E, Girolami A. Decrease of fibrinogen in patients with peripheral atherosclerotic disease by ticlopidine. Arzneimittel‐Forschung 1991;41(4):414‐6. [PubMed] [Google Scholar]
Ranke 1992 {published data only}
- Ranke C, Creutzig A, Luska G, Wagner H‐H, Galanski M, Frolich JC, et al. [Comparison of 50 mg and 900 mg/day acetylsalicylic acid for prevention of recurrence after percutaneous transluminal angioplasty of the lower extremities: results of the LARA study]. Vasa.Supplementum 1992;35:42‐5. [PubMed] [Google Scholar]
Ranke 1993 {published data only}
- Ranke C, Creutzig A, Luska G, Wagner HH, Galanski M, Bode‐Boger S, et al. Dose‐dependent side effects of acetylsalicylic acid therapy. Results of a prospective randomized clinical study in patients with peripheral arterial occlusive disease. Medizinische Klinik 1993;88(10):571‐6. [PubMed] [Google Scholar]
Ranke 1994 {published data only}
- Ranke C, Creutzig A, Luska G, Wagner HH, Galanski M, Bode‐Boger S, et al. Controlled trial of high‐ versus low‐dose aspirin treatment after percutaneous transluminal angioplasty in patients with peripheral vascular disease. The Clinical Investigator 1994;72(9):673‐80. [DOI] [PubMed] [Google Scholar]
Regenthal 1991 {published data only}
- Regenthal R, Voigt H, Reuter W, Preiss R. [The effect of oral trapidil therapy on clinical and hemorheological parameters in arteriosclerosis obliterans in comparison to pentoxifylline‐‐a pilot study]. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1991;46(6):185‐90. [PubMed] [Google Scholar]
Rossini 1998 {published data only}
- Rossini A, Tascino C, Costa F. Heparan sulphate in association with indobufen in the treatment of chronic peripheral obliterative arteriopathy of the lower extremities. Controlled clinical trial. Minerva Cardioangiologica 1998;46(11):457‐69. [PubMed] [Google Scholar]
Roztocil 1989 {published data only}
- Roztocil K, Oliva I, Prerovsky I, Linhart J. The effect of hydroxyethylrutoside and its combination with acetylsalicylic acid in patients with obliterative atherosclerosis. Cor et Vasa 1989;31(2):128‐33. [PubMed] [Google Scholar]
Rudofsky 1987 {published data only}
- Rudofsky G, Meyer P, Hirche H, Altenhoff B, Lohman A. [Recognition and control of early arterio‐sclerotic vascular lesions]. Vasa.Supplementum 1987;20:165‐8. [PubMed] [Google Scholar]
Schoop 1987 {published data only}
- Schoop W. Progression and regression of peripheral occlusive arterial disease: spontaneous course ‐ ASS‐ASS+dipyridamol. Vasa.Supplementum 1987;20:62‐3. [Google Scholar]
Schweizer 2003 {published data only}
- Schweizer J, Kirch W, Koch R, Muller A, Hellner G, Forkmann L. Use of abciximab and tirofiban in patients with peripheral arterial occlusive disease and arterial thrombosis. Angiology 2003;54(2):155‐61. [DOI] [PubMed] [Google Scholar]
Singer 2006 {published data only}
- Singer E, Imfeld S, Hoffmann U, Buschmann I, Labs KH, Jaeger KA. Aspirin in peripheral arterial disease: Breakthrough or pitfall?. Vasa 2006;35(3):174‐7. [DOI] [PubMed] [Google Scholar]
Smith 1981 {published data only}
- Smith RS, Warren DJ. Effect of nicotinic acid and dipyridamole on tissue blood flow in peripheral vascular disease. Pharmatherapeutica 1981;2(9):597‐600. [PubMed] [Google Scholar]
Stiegler 1984 {published data only}
- Stiegler H, Hess H, Mietaschk A, Trampisch HJ, Ingrisch H. Effect of ticlopidine on peripheral obliterating arteriopathy. Deutsche Medizinische Wochenschrift 1984;109(33):1240‐3. [DOI] [PubMed] [Google Scholar]
Tepe 2007 {published data only}
- Tepe G. Mirror Trial ‐ Follow‐Up Management of Peripheral Arterial Intervention With Clopidogrel. http://clinicaltrials.gov/show/NCT00163267 2007.
Topol 2000 {published data only}
- Topol EJ, Easton JD, Amarenco P, Califf R, Harrington R, Graffagnino C, et al. Design of the blockade of the glycoprotein IIb/IIIa receptor to avoid vascular occlusion (BRAVO) trial. American Heart Journal 2000;139(6):927‐33. [DOI] [PubMed] [Google Scholar]
Warfarin 2007 {published data only}
- Warfarin Antiplatelet Vascular Evaluation Trial Investigators, Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. [see comment]. New England Journal of Medicine 2007;357(3):217‐27. [DOI] [PubMed] [Google Scholar]
Wilhite 2003 {published data only}
- Wilhite DB, Comerota AJ, Schmieder FA, Throm RC, Gaughan JP, Rao AK. Managing PAD with multiple platelet inhibitors: the effect of combination therapy on bleeding time. Journal of Vascular Surgery 2003;38(4):710‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Delaimy 2004
- Al‐Delaimy WK, Merchant AT, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. American Journal of Medicine 2004;116(4):236‐40. [DOI] [PubMed] [Google Scholar]
Aronow 1994
- Aronow WS, Ahn C. Prevalence of coexistence of coronary artery disease, peripheral arterial disease, and atherothrombotic brain infarction in men and women > or = 62 years of age. American Journal of Cardiology 1994;74(1):64‐5. [DOI] [PubMed] [Google Scholar]
ATC 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324(7329):71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aung 2007
- Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC. Lipid‐lowering for peripheral arterial disease of the lower limb. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000123.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Awtry 2000
- Awtry EH, Loscalzo J. Aspirin. Circulation 2000;101(10):1206‐18. [DOI] [PubMed] [Google Scholar]
Balkau 1994
- Balkau B, Vray M, Eschwege E. Epidemiology of peripheral arterial disease. Journal of Cardiovascular Pharmacology 1994;23(Suppl 3):S8‐16. [PubMed] [Google Scholar]
Basili 2010
- Basili S, Raparelli V, Vestri A, Tanna GL, Violi F. Comparison of efficacy of antiplatelet treatments for patients with claudication. A meta‐analysis. Thrombosis & Haemostasis 2010;103(4):766‐73. [DOI] [PubMed] [Google Scholar]
Belch 2003
- Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Archives of Internal Medicine 2003;163(8):884‐92. [DOI] [PubMed] [Google Scholar]
Bennett 1999
- Bennett CL, Davidson CJ, Raisch DW, Weinberg PD, Bennett RH, Feldman MD. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Archives of Internal Medicine 1999;159(21):2524‐8. [DOI] [PubMed] [Google Scholar]
Berger 2009
- Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta‐analysis of randomized trials. JAMA 2009;301(18):1909‐19. [DOI] [PubMed] [Google Scholar]
Bhatt 2002
- Bhatt DL, Bertrand ME, Berger PB, L'Allier PL, Moussa I, Moses JW, et al. Meta‐analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. Journal of the American College of Cardiology 2002;39(1):9‐14. [DOI] [PubMed] [Google Scholar]
Casella 2003
- Casella G, Ottani F, Pavesi PC, Sangiorgio P, Rubboli A, Galvani M, et al. Safety and efficacy evaluation of clopidogrel compared to ticlopidine after stent implantation: an updated meta‐analysis. Italian Heart Journal 2003;4(10):677‐84. [PubMed] [Google Scholar]
Cordoba 2010
- Cordoba G, Schwartz, L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341:c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Criqui 1992
- Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. New England Journal of Medicine 1992;326(6):381‐6. [DOI] [PubMed] [Google Scholar]
Davi 2007
- Davì G, Patrono C. Platelet activation and atherothrombosis. New England Journal of Medicine 2007;357(24):2482‐94. [DOI] [PubMed] [Google Scholar]
Fagher 1993
- Fagher B, Persson S, Persson G, Larsson H. Blood viscosity during long‐term treatment with ticlopidine in patients with intermittent claudication. A double‐blind study. Angiology 1993;44(4):300‐6. [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499‐533. [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1991;20(2):384‐92. [DOI] [PubMed] [Google Scholar]
Fowkes 2008
- Ankle Brachial Index Collaboration: Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA 2008;300(2):197‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 1995
- Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta‐analysis. JAMA 1995;274(12):975‐80. [PubMed] [Google Scholar]
Hiatt 2002b
- Hiatt WR. Preventing atherothrombotic events in peripheral arterial disease: the use of antiplatelet therapy. Journal of Internal Medicine 2002;251(3):193‐206. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HPSCG 2007
- Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol‐lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high‐risk conditions. Journal of Vascular Surgery 2007;45(4):645‐54; discussion 653‐4. [DOI] [PubMed] [Google Scholar]
Jonason 1985
- Jonason T, Ringqvist I. Mortality and morbidity in patients with intermittent claudication in relation to the location of the occlusive atherosclerosis in the leg. Angiology 1985;36(5):310‐4. [DOI] [PubMed] [Google Scholar]
Kannel 1970
- Kannel WB, Skinner JJ Jr, Schwartz MJ, Shurtleff D. Intermittent claudication. Incidence in the Framingham Study. Circulation 1970;41(5):875‐83. [DOI] [PubMed] [Google Scholar]
Leng 1993
- Leng GC, Fowkes FGR. The epidemiology of peripheral arterial disease. Vascular Medicine Review 1993;4(1):5‐18. [Google Scholar]
Leng 1996
- Leng GC, Lee A, Fowkes F, Whiteman M, Dunbar J, Housley E, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral artery disease in the general population. International Journal of Epidemiology 1996;25(6):1172‐81. [DOI] [PubMed] [Google Scholar]
MacGregor 1999
- MacGregor AS, Price JF, Hau CM, Lee AJ, Carson MN, Fowkes FG. Role of systolic blood pressure and plasma triglycerides in diabetic peripheral arterial disease. The Edinburgh Artery Study. Diabetes Care 1999;22(3):453‐8. [DOI] [PubMed] [Google Scholar]
Meade 2002
- Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: randomised controlled trial. BMJ 2002;325(7373):1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moloney 1993
- Moloney BA. An analysis of the side effects of ticlopidine. In: Hass WK, Easton JD editor(s). Ticlopidine, Platelets and Vascular Disease. New York: Springer, 1993:117‐39. [Google Scholar]
Newman 1993
- Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation 1993;88(3):837‐45. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG on behalf of the TASC II Working Group. Inter‐Society Consensus for the Management of Peripheral Arterial Disease (TASC II). European Journal of Vascular and Endovascular Surgery 2007;33(Suppl 1):S1‐70. [DOI] [PubMed] [Google Scholar]
Patrono 2004
- Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet‐active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(Suppl 3):234S‐246S. [DOI] [PubMed] [Google Scholar]
Ratti 1998
- Ratti S, Quarato P, Casagrande C, Fumagalli R, Corsini A. Picotamide, an antithromboxane agent, inhibits the migration and proliferation of arterial myocytes. European Journal of Pharmacology 1998;355(1):77‐83. [DOI] [PubMed] [Google Scholar]
Robless 2003
- Robless P, Mikhailidis DP, Stansby GP. Antiplatelet agents for intermittent claudication. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenberg 1990
- Rosenberg L, Palmer JR, Shapiro S. Decline in the risk of myocardial infarction among women who stop smoking. The New England Journal of Medicine 1990;322(4):213‐7. [DOI] [PubMed] [Google Scholar]
Schror 2000
- Schror K. Ticlopidine and Clopidogrel. In: Ferguson JJ III, Chronos NAF, Harrington RA editor(s). Antiplatelet Therapy in Clinical Practice. London, England: Martin Dunitz, 2000:93‐111. [Google Scholar]
Selvin 2004
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999‐2000. Circulation 2004;110(6):738‐43. [DOI] [PubMed] [Google Scholar]
Singer 2008
- Singer DR, Kite A. Management of hypertension in peripheral arterial disease: does the choice of drugs matter?. European Journal of Vascular and Endovascular Surgery 2008;35(6):701‐8. [DOI] [PubMed] [Google Scholar]
Steg 2007
- Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297(11):1197‐206. [DOI] [PubMed] [Google Scholar]
Sudlow 2009
- Sudlow CLM, Mason G, Maurice JB, Wedderburn CJ, Hankey GJ. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD001246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueno 2011
- Ueno M, Kodali M, Tello‐Montoliu A, Angiolillo DJ. Role of platelets and antiplatelet therapy in cardiovascular disease. Journal of Atherosclerosis and Thrombosis 2011;18(6):431‐42. [DOI] [PubMed] [Google Scholar]
Varga‐Szabo 2008
- Varga‐Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arteriosclerosis, Thrombosis and Vascular Biology 2008;28(3):403‐12. [DOI] [PubMed] [Google Scholar]
Watson 2008
- Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000990.pub2] [DOI] [PubMed] [Google Scholar]


