Abstract
Influence of vitamin A (retinol) on growth depends on its sequential oxidation to retinal and then to retinoic acid (RA), producing a ligand for RA receptors essential in development of specific tissues. Genetic studies have revealed that aldehyde dehydrogenases function as tissue-specific catalysts for oxidation of retinal to RA. However, enzymes catalyzing the first step of RA synthesis, oxidation of retinol to retinal, remain unclear because none of the present candidate enzymes have expression patterns that fully overlap with those of aldehyde dehydrogenases during development. Here, we provide genetic evidence that alcohol dehydrogenase (ADH) performs this function by demonstrating a role for Adh3, a ubiquitously expressed form. Adh3 null mutant mice exhibit reduced RA generation in vivo, growth deficiency that can be rescued by retinol supplementation, and completely penetrant postnatal lethality during vitamin A deficiency. ADH3 was also shown to have in vitro retinol oxidation activity. Unlike the second step, the first step of RA synthesis is not tissue-restricted because it is catalyzed by ADH3, a ubiquitous enzyme having an ancient origin.
Retinoic acid (RA) signaling depends on enzymes capable of catalyzing the two-step conversion of retinol to RA (1), with RA then functioning as a ligand for RA receptors known to control vertebrate growth and development (2). Enzymes essential for the first step of RA synthesis, oxidation of retinol to retinal, remain unclear, although several candidates exist within the alcohol dehydrogenase (ADH) and short-chain dehydrogenase/reductase (SDR) families (1). The mammalian ADH family (3), a subset of the medium-chain dehydrogenase/reductase superfamily (4), consists of five classes of cytosolic enzymes encoded by a tightly linked cluster of genes tandemly organized in the same transcriptional orientation on the same chromosome (5). Class I (ADH1), class II (ADH2), and class IV (ADH4) are reported to catalyze the oxidation of retinol to retinal in vitro and may thus contribute to RA synthesis (6–9). The SDR family (4) includes microsomal enzymes reported to convert retinol to retinal in vitro that are hypothesized to participate in the RA biosynthetic pathway; i.e., RoDH1 and RoDH2 (10–12), CRAD1 and CRAD2 (13,14), and RDH5 (15, 16). Because conclusive genetic evidence now exists that retinaldehyde dehydrogenase 2 (RALDH2) catalyzes the second step of RA synthesis during development, metabolism of retinal to RA (17, 18), it seems appropriate that enzymes producing retinal should be coexpressed with RALDH2 (19, 20) and with the related RA-generating aldehyde dehydrogenases, RALDH1 and RALDH3 (21–23). However, the tissue-specific and developmental stage-specific patterns of expression for the above-mentioned ADHs (24–26) and SDRs (15, 26) do not fully overlap with expression of the RALDHs, either individually or collectively, although a partial overlap occurs. Thus, a retinol-oxidizing enzyme whose expression would fully overlap with the RALDHs, or fill the missing gaps, has not yet been described.
Genetic studies initiated on ADHs and SDRs have provided some evidence of functions in retinoid metabolism. Adh4−/− mice are viable but suffer increased postnatal lethality during gestational vitamin A deficiency (VAD) (27). Thus, ADH4 is an essential retinal-generating enzyme for survival during VAD, a condition that may be commonly encountered by animals in the wild. Because Adh4−/− mice demonstrate no reduction in reproductive ability or growth when fed a vitamin A-sufficient diet, at least one additional enzyme must function to provide retinal for RA synthesis during development. Further studies have demonstrated that Adh1−/− mice are viable but deficient in metabolism of a dose of retinol to RA (28). Rdh5−/− mice are viable but suffer a mild visual defect, a delay in dark adaptation, thus consistent with its proposed role in metabolism of 11-cis-retinol to 11-cis-retinal to produce the visual chromophore (29). Humans containing RDH5 mutations suffer fundus albipunctatus, a rare form of stationary night blindness (30), but no role in RA synthesis has been shown for RDH5.
Class III ADH (ADH3) is a ubiquitously expressed enzyme (24, 25, 31) with an ancient origin (32, 33) that has not been associated with retinol dehydrogenase activity (6–8). Instead, ADH3 has been suggested to function as a glutathione-dependent formaldehyde dehydrogenase in both unicellular and multicellular organisms (34, 35). However, we reported that Adh3−/− mice exhibit only a 30% reduction in the LD50 value of formaldehyde (28), suggesting that other mechanisms exist for clearance of formaldehyde and hinting that ADH3 may have another function in vertebrate animals. We now report that mouse ADH3 functions as a previously unrecognized cytosolic retinol dehydrogenase contributing to RA synthesis. This finding fills the gap in our understanding of how RALDHs obtain retinal for RA generation because ADH3 is expressed ubiquitously and can thus supply substrate to all RALDHs.
Materials and Methods
Mouse Strains.
Mice carrying homozygous null mutations of Adh1 and Adh3 (28) as well as Adh4 (27) have been described. Adh1−/−, Adh3−/−, Adh4−/−, and wild-type mice were propagated on a standard mouse diet (Purina 5015 Mouse Chow containing 30 units/g vitamin A) unless otherwise specified. All of these mice have the same genetic background. Care of animals was in accordance with institutional guidelines.
Vitamin A Supplementation.
Mice were propagated on either Purina 5015 Standard Mouse Chow, which contains the standard amount of vitamin A (30 units/g), or Purina 5755 Basal Diet supplemented with additional retinyl acetate to bring the total vitamin A concentration to 300 units/g (all in the form of retinyl acetate, which is quickly hydrolyzed to retinol in the digestive tract). Groups of adult female mice that had been placed on each of these diets for 2 weeks were mated with males to generate offspring while still on their respective diets.
Analysis of Retinol Metabolism in Vivo.
Retinol was administered essentially as described (36). all-trans-Retinol (Sigma) was dissolved in acetone/Tween 20/water (0.25:5:4.75 vol/vol) and a dose of 50 mg/kg or vehicle control was injected orally to female mice. After 2 h, blood was collected and stored at −20°C until analysis. Serum (200 μl) was extracted with 2 ml of methanol/acetone (50:50 vol/vol). After centrifugation at 10,000 × g for 10 min at 4°C, the organic phase was evaporated under vacuum and the residue was dissolved in 200 μl of methanol/dimethyl sulfoxide (50:50 vol/vol). Samples were analyzed by HPLC to quantitate levels of all-trans-RA and all-trans-retinol by using standards for each of these retinoids (Sigma). Reversed-phase HPLC analysis was performed by using a MICROSORB-MVTM 100 C18 column (4.5 × 250 mm) (Varian) at a flow rate of 1 ml/min. Mobile phase consisted of 0.5 M ammonium acetate/methanol/acetonitrile (25:65:10 vol/vol) (solvent A) and acetonitrile (solvent B). The gradient composition was (only solvent B is mentioned): 0% at the time of injection; 30% at 1 min; 35% at 14 min; 100% at 16 min. UV detection was performed at 340 nm.
Measurement of ADH Retinol Oxidation Activity in Vitro.
Mouse ADH1, ADH3, and ADH4 were expressed in Escherichia coli BL21 as glutatione S-transferase fusion proteins as described (31). Purification of ADH-glutatione S-transferase fusion proteins was performed by incubation of cell homogenates with glutathione-Sepharose 4B (Amersham Pharmacia) at 20°C for 15 h, and ADH was eluted by digestion with thrombin (10 units/mg protein). During the purification procedure, enzyme activities were determined spectrophotometrically (37) in 0.1 M glycine/NaOH (pH 10.5) with 0.3 mM NAD and 10 mM ethanol for ADH1, 2.4 mM NAD and 1 mM octanol for ADH3, or 2.4 mM NAD and 2.5 M ethanol for ADH4.
Retinol assays were performed at 37°C in 1 ml of 0.1 M phosphate buffer, pH 7.5/40 mM NaCl/4 mM NAD/4 mg/ml BSA/30 μM all-trans-retinol (7 μl of 1.2 mg/ml stock in acetone). The assay was initiated by addition of enzyme except for controls, which had no enzyme added. After 30 min, 20 μl of 2 M NH2OH (pH 6.5) was added to stop the reaction and convert retinal to the oxime to stabilize it for HPLC analysis as described (38). Samples were incubated at 25°C for another 30 min, diluted with 2 ml of methanol, and extracted twice with 4 ml of hexane. The hexane phases were combined and evaporated under vacuum. Residues were resuspended in 150 μl of hexane and injected into the HPLC system for analysis. As a standard, all-trans-retinal (Sigma) was similarly treated with NH2OH to produce all-trans-retinaloxime. Analysis of all-transretinaloxime in the samples was performed on a normal-phase isocratic HPLC system with a MICROSORB-MVTM 100 Silica column (4.5 × 250 mm) (Varian). Mobile phase consisted of 95% hexane and 5% ethyl acetate at a flow rate of 2 ml/min. UV detection was performed at 325 nm.
Vitamin A Deficiency.
Gestational VAD in mice was induced by a modification of previous methods (27). For each mouse strain, original parental mice (three mating pairs each) were placed on Purina VAD diet 5822 (vitamin A <0.22 units/g) at the beginning of mating, and resulting offspring were maintained on this diet. Congenitally VAD F1 generation females (8 for each strain) were mated at 6 weeks of age (to males maintained on standard mouse chow to remain fertile), thus producing congenitally VAD F2 generation mice. For both rounds of matings, females were separated from males before birth to ensure that they did not again become pregnant during postnatal development of their offspring. Serum all-trans-retinol was quantitated by HPLC as indicated above.
Results
Survival and Growth of Adh3−/− Mice.
When the Adh3−/− mouse line was initially described it was observed that homozygous mice survived and reproduced and no indication of a significant reduction in survival had yet been noticed, but only 27 progeny were examined (28). Subsequently, Adh3−/− adult mice were noticed to weigh significantly less than wild-type (WT) mice suggesting a growth deficiency (L.D., G.D., unpublished data). To investigate this further, we compared the generation, survival, and growth of numerous Adh3−/− mice with two other ADH null mutant mouse strains (Adh1−/− and Adh4−/−) and with WT mice. Adh3−/− mice exhibited smaller litter sizes than WT (6.95 vs. 8.47), lower postnatal survival (85% vs. 99%), and smaller body weights at 14 weeks of age (24.7 g vs. 34.3 g) (Table 1). Adh1−/− and Adh4−/− mice showed no significant difference with WT for any parameters examined (Table 1). Thus, targeted disruption of Adh3 has a significant negative effect on survival and growth.
Table 1.
Generation, survival, and growth of ADH-deficient mice
| Genotype | Litters | Pups born | Pups/litter | Postnatal mortality | % survival, no. | Weight at 14 weeks, g |
|---|---|---|---|---|---|---|
| WT | 17 | 144 | 8.47 ± 0.47 | 1 | 99.3 (143) | 34.3 ± 1.2 |
| Adh1−/− | 17 | 151 | 8.88 ± 0.49 | 7 | 95.4 (144) | 34.4 ± 3.2 |
| Adh3−/− | 17 | 121 | 6.95 ± 0.51* | 18 | 85.1 (103)** | 24.7 ± 0.8** |
| Adh4−/− | 17 | 166 | 9.76 ± 0.63 | 3 | 98.2 (163) | 38.2 ± 3.4 |
All mice were propagated on standard mouse chow. Reported postnatal mortality occurred between birth and weaning (postnatal day 19); % survival refers to mice alive at weaning. Values are mean ± SE; for determination of body weight in 14-week-old male mice (n = 14).
, P < 0.05;
, P < 0.001 (Student's t test, Adh3−/− vs. WT).
Retinol Rescue of Adh3−/− Growth Deficiency.
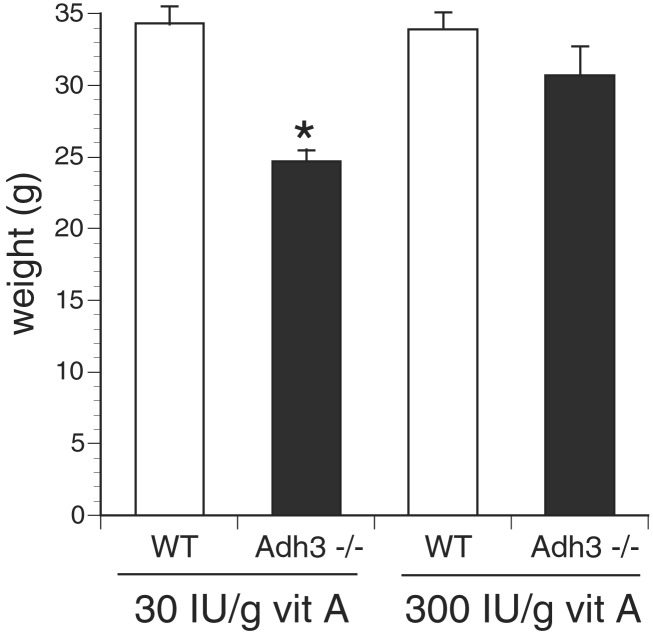
To determine whether the growth deficiency of Adh3−/− mice could be rescued by retinol treatment, we compared Adh3−/− and WT mice generated on standard mouse chow with mice generated on a retinol-supplemented diet containing 10-fold higher vitamin A than standard mouse chow. Whereas standard mouse chow allowed growth of Adh3−/− mice to achieve only 72% the weight of WT mice (24.7 ± 0.8 g vs. 34.3 ± 1.2 g at 14 weeks of age), the retinol-supplemented diet increased growth of Adh3−/− mice nearly to normal compared with WT (30.7 ± 2.0 g vs. 33.9 ± 1.2 g at 14 weeks of age; no statistically significant difference) (Fig. 1). The growth of WT mice was not significantly changed by retinol supplementation, indicating that the effect was specific for mice lacking Adh3. Thus, retinol treatment can significantly rescue the growth deficiency phenotype of Adh3−/− mice. These studies establish that ADH3 is essential for optimal usage of standard dietary levels of retinol to promote normal growth, and that other enzymes cannot replace this function unless provided supraphysiological amounts of retinol.
Figure 1.
Retinol rescue of Adh3−/− growth deficiency. Mice were propagated for one generation on either Purina 5015 Standard Mouse Chow containing the standard amount of vitamin A (30 units/g) or Purina 5755 Basal Diet supplemented with additional retinyl acetate to bring the total vitamin A concentration to 300 units/g. Reported are the weights of male offspring at 14 weeks of age. All values are mean ± SEM (n = 14); *, P < 0.001 (Student's t test, Adh3−/− vs. wild-type, both on standard mouse chow).
Metabolism of Retinol to RA in Vivo by ADH3.
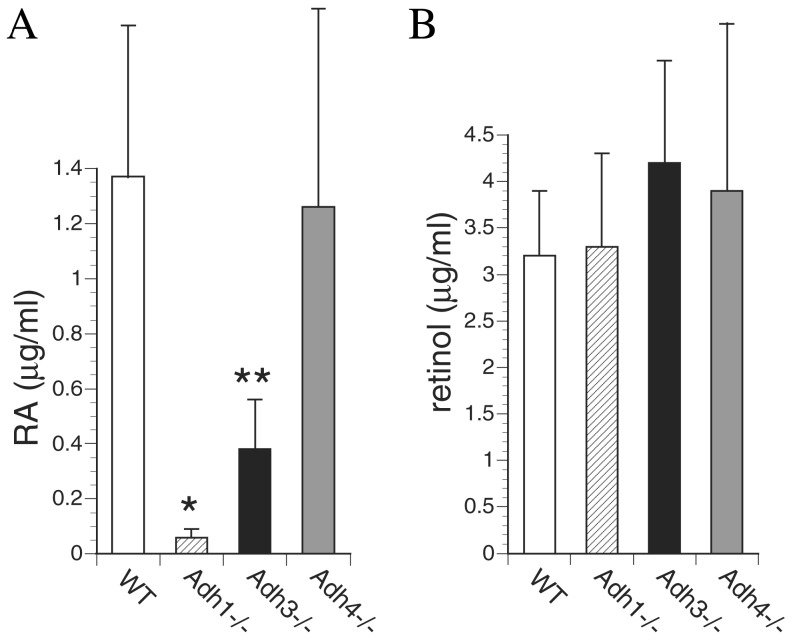
Because ADH3 has not been reported to have retinol oxidation activity, we tested all strains of mice for metabolism of a dose of retinol to RA, the signaling molecule needed for growth. Mice were treated orally with a 50 mg/kg dose of all-trans-retinol and 2 h later all-trans-RA was quantitated in serum (36). Whereas WT mice generated high levels of serum all-trans-RA (1.37 μg/ml) in response to this dose of retinol, we observed that Adh1−/− mice had a 23-fold reduction (0.06 μg/ml), Adh3−/− mice had a 3.6-fold reduction (0.38 μg/ml), and Adh4−/− mice had a small reduction (1.26 μg/ml) that was not statistically significant (Fig. 2A). These findings indicate that ADH3 contributes significantly to RA generation in vivo, and under these conditions makes a larger contribution than ADH4 perhaps because of its ubiquitous expression compared with the much more limited expression pattern of ADH4. ADH4 is not expressed in liver or intestine and instead is limited to epithelia of the stomach, esophagus, skin, adrenal, and reproductive tract (31, 39–41). The observed major role of ADH1 in metabolism of a dose of retinol to RA is consistent with its expression at very high levels in liver, intestine, and several other organs (31). To ensure that all strains had been successfully treated, we also examined retinol. Serum all-trans-retinol levels in treated mice ranged from 3.2 to 4.2 μg/ml among the five strains showing no significant difference (Fig. 2B). Because untreated mice generally have serum all-trans-retinol levels between 0.3 and 0.4 μg/ml (see below; Fig. 3D), the treatment resulted in an approximate 10-fold increase for each strain.
Figure 2.
Effect of Adh genotype on metabolism of a dose of retinol to RA. (A) For WT mice and each Adh null mutant strain indicated, all-trans-RA levels were quantitated by HPLC in serum 2 h after a 50 mg/kg oral dose of all-trans-retinol; all values are from adult female mice (n = 4). (B) Serum all-trans-retinol levels quantitated by HPLC are shown for the same mice examined above. All values are mean ± SEM. *, P < 0, 03; **, P < 0.05; (Student's t test, null mutant vs. WT).
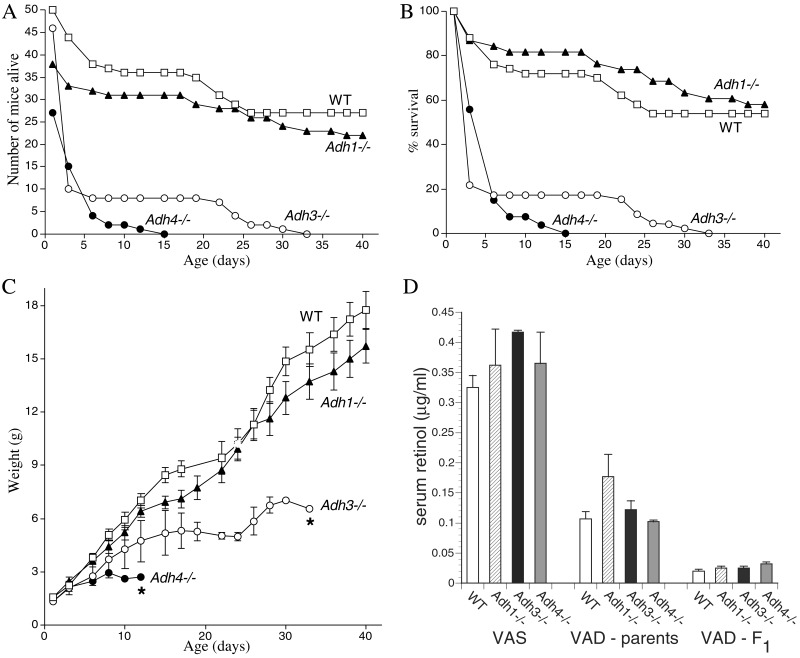
Figure 3.
Adh3−/− mice undergo postnatal lethality during gestational VAD. (A) After exposure to VAD, the number of congenitally VAD F2 generation offspring born for WT mice and each Adh null mutant strain is shown, plus the numbers surviving until postnatal day 40. (B) The previous data are plotted as the percent survival for each mouse strain. Less than 20% survival was observed for Adh3 and Adh4 mutants by postnatal day 6. (C) The weight gain for the WT and null mutant mice above is shown (*, all mice had died by the day indicated). (D) Depletion of serum retinol in WT and Adh null mutant mice during gestational VAD is shown. Serum all-trans-retinol levels were quantitated by HPLC. Control values are shown for 14-week-old vitamin A-sufficient (VAS) female mice (n = 3) maintained on standard mouse chow. Values indicated as VAD-parents refer to the original female parents used to begin the VAD studies (n = 3); these mice were placed on the VAD diet at 6 weeks of age, mated to produce one litter; then at 14weeks of age, serum retinol was measured. Values indicated as VAD-F1 refer to the F1 generation of congenitally VAD female offspring (n = 8) of the original VAD female parents; these females were mated at 6 weeks of age to produce the F2 generation mice described in A–C; then at 14 weeks of age, serum retinol was measured.
Oxidation of Retinol to Retinal by ADH3 in Vitro.
We addressed the ability of ADH3 to oxidize retinol to retinal in vitro. Because this ability had not been previously demonstrated, we investigated the methods used. Previous measurements of ADH retinol oxidation activity relied on the relatively easy spectrophotometric measurement of retinal production (6–9). The sensitivity of this assay is much lower than HPLC quantitation of all-trans-retinal used to measure retinol oxidation by microsomal SDRs, which have much lower activity than ADH1 and ADH4 (11, 13, 14, 16). Thus, in vitro assays of retinol oxidation by mouse ADH1, ADH3, and ADH4 were performed by HPLC quantitation of all-trans-retinal production. With this assay we did detect retinol oxidation activity by ADH3 at the rate of 105 pmol⋅min−1⋅mg−1 (Table 2).
Table 2.
Oxidation of all-trans-retinol by mouse ADHs
| Enzyme | all-trans-retinal generated, pmol⋅min−1⋅mg protein−1 |
|---|---|
| ADH1 | 150,500 ± 5,500 |
| ADH3 | 105 ± 7 |
| ADH4 | 422,000 ± 15,000 |
Quantitation was by HPLC. Values are mean ± SE (n = 4). Control reactions with no enzyme yielded a value of 25 ± 5 pmol/min (n = 4) which was subtracted from reported values.
As expected we found that ADH1 and ADH4 had relatively high activities of 150,500 and 422,000 pmol⋅min−1⋅mg−1, respectively (Table 2). Previous measurements of human and rat ADH retinol oxidation by using the spectrophotometric assay indicate that ADH1 activity ranges from 30,000 to 300,000 pmol⋅min−1⋅mg−1, whereas ADH4 activity ranges from 110,000 to 1,675,000 pmol⋅min−1⋅mg−1 (6–9). Thus, our assays with HPLC quantitation show comparable results for mouse ADH1 and ADH4. Human ADH3 reportedly has no measurable activity with retinol, but the limit of detection for measurement of retinal spectrophotometrically was indicated as <0.08 min−1 or 2,000 pmol⋅min−1⋅mg−1 (7). It is now clear that the amount of retinol activity we observe for ADH3 could not have been detected in previous studies.
Effect of Vitamin A Deficiency on Survival and Growth.
We examined how Adh3−/− mice would perform in comparison with WT, Adh1−/−, and Adh4−/− mice when subjected to gestational VAD. Because it is difficult to induce VAD in mice, all mice were mated for two generations on a VAD diet as described (27). No increase in mortality relative to mice maintained on normal chow was noticed in the original parents or the F1 generation up to 14 weeks of age for any of the mouse strains. In the F2 generation, 80% of Adh3−/− mice died between birth and postnatal day 3 (P3), and 100% lethality was observed by P33, thus indicating that ADH3 does provide protection against VAD (Fig. 3 A and B). Adh4−/− mice also behaved poorly under VAD conditions in the F2 generation, displaying 100% lethality by P15, as expected from previous VAD studies (27). In contrast, WT and Adh1−/− mice both exhibited approximately 60% survival at P40 (Fig. 3 A and B).
For all strains of mice, signs of growth deficiency were noticed in the F2 generation, but this deficiency was most pronounced in mice lacking either ADH3 or ADH4. Adh3−/− mice failed to achieve body weights beyond 6 g, whereas Adh4−/− mice failed to grow beyond 3 g (Fig. 3C). Growth of WT and Adh1−/− mice was much less affected by VAD, because body weights at P40 were 16–18 g for each strain (Fig. 3C), 30–35% lower relative to age-matched mice maintained on standard mouse chow (data not shown). Thus, in contrast to ADH3 and ADH4, a lack of ADH1 did not have an effect on growth during VAD. No additional mortality was noticed in WT and Adh1−/− mice maintained until P80, and body weight maximized by P50 at 18–19 g. Among the mice that died from each strain we consistently observed additional signs of systemic VAD, i.e., crusty rings on their eyelids (dryness of the conjunctiva), loss of hair, and lethargy. These experiments demonstrate that ADH3 and ADH4 each play essential roles in survival and growth during VAD, but that ADH1 is not essential, because Adh1−/− and WT mice had similar survival and growth during VAD.
To verify that each mouse strain had been successfully subjected to VAD, we measured retinol in the female mice used to generate the F2 generation. Serum all-trans-retinol levels in 14-week-old control females maintained on standard mouse chow ranged from 0.3 to 0.4 μg/ml for the various strains, whereas 14-week-old females maintained on the VAD diet for the last 8 weeks (original parents) had levels ranging from 0.1 to 0.17 μg/ml, and 14-week-old congenitally VAD F1 generation females (parents of F2 generation described above) all had levels of about 0.03 μg/ml (Fig. 3D). Thus, all five strains of mice responded essentially equally to VAD in terms of reduction in the level of serum all-trans-retinol.
Discussion
The findings above establish that ADH3 performs an essential role in the retinoid-signaling pathway, i.e., generation of retinal to be used as a substrate by any of the RALDHs to produce RA. ADH3 fits this role quite nicely because it, unlike other ADHs or SDRs, is expressed ubiquitously. In mouse embryos from embryonic day 7.5 (E7.5) to E9.0, the expression pattern of the RA-generating enzyme RALDH2 is very similar to the RA localization pattern, i.e., in trunk mesoderm up to the junction with the hindbrain and in the optic vesicle (17–20). Raldh2−/− mice totally lack RA in the trunk and have only a small amount of RA remaining in the eye region, thus demonstrating that RALDH2 is indeed the major RA-generating enzyme at this stage of development (17, 18). Because ADH3 mRNA has been detected at high levels in all cells at E7.5–E9.5 by whole-mount in situ hybridization (24), it could produce retinal for all tissues expressing RALDH2 or any other RA-generating enzyme. ADH3 mRNA remains ubiquitous in late-gestation mouse embryos (25), and by adulthood ADH3 protein is easily detected by Western blotting in all mouse tissues analyzed (31). In cells that do not express RALDH2 or another RA-generating enzyme, the retinal produced by ADH3 would not accumulate because it would simply be in equilibrium with retinol due to the reversible nature of this reaction.
It is clear that other retinol-oxidizing enzymes must exist or Adh3−/− mice would have suffered completely penetrant mid-gestation lethality as Raldh2−/− mice do (17, 18). ADH4 mRNA has been detected in mouse embryos at stages E7.5–E9.5, but it is not expressed in the optic vesicle, and expression in trunk mesoderm is centered at a more posterior location than that seen for RALDH2 mRNA (24). As for the other candidate enzymes, either very limited or no overlap occurred with RALDH2 mRNA from E7.5–E9.5 as summarized. ADH1 mRNA is first observed at E9.5 limited to the mesonephric ducts (24); RDH5 mRNA at E8.5 is localized in neuroepithelia (15); reverse transcription–PCR studies have shown that ADH1 plus CRAD1 mRNAs are first detected at E9.5, and that RoDH plus CRAD2 mRNAs are undetectable from E6.5 to E9.5, and further that ADH4 plus RDH5 mRNAs are detected from E6.5 to E9.5 with RALDH2 mRNA detected from E7.5 to E9.5 (26). Thus, whereas additional retinol-oxidizing enzymes exist, a combination of them may be needed to approach the efficiency of ADH3 for providing retinal throughout the body for RA synthesis. In fact, the efficiency of ADH3 is not quite achieved by all remaining enzymes, because we have shown here that Adh3−/− mice suffer reduced survival and growth deficiency, requiring supraphysiological levels of retinol to gain back some of these losses.
Efficiency here is not measured in terms of how much activity the enzyme has, for ADH3 retinol activity is lower than that of ADH1 or ADH4, but rather in terms of whether it is expressed properly (as ADH3 is) to provide the small amount of retinal needed to fuel local production of RA wherever and whenever needed. Because RA is usually present in tissues at only nanomolar quantities (26, 36), and because these quantities are sufficient to regulate RA receptors (2), the level of ADH3-catalyzed retinol oxidation observed here could be sufficient to generate this amount of RA. The activity of 105 pmol⋅min−1⋅mg−1 we observe for ADH3 is comparable with the 115 pmol⋅min−1⋅mg−1 reported for partially purified RoDH, which has been hypothesized to play a significant role in RA synthesis (11). Other SDRs (CRAD1, CRAD2, and RDH5) overexpressed in cell lines have been reported to have all-trans-retinol activities between 20 and 200 pmol⋅min−1⋅mg−1 of cell extract (13, 14, 16). Although it is difficult to compare the activities of ADHs directly with SDRs because of differences in purity, we conclude that ADH3 has retinol oxidation activity that is much less than the oxidation activity of other ADHs, but in the same order of magnitude as the activity seen for microsomal SDRs. However, the physiological impact of SDRs on RA synthesis during development may be less than the impact of ADH3, because they lack the ubiquitous expression pattern that ADH3 possesses (however, see Note Added in Proof).
It was hypothesized that ADHs may not be able to contribute significantly to RA synthesis because the vast majority of retinol is physiologically bound to cellular retinol-binding protein type I (CRBPI), and it was reported that ADH4 had no detectable activity with CRBPI-retinol, whereas microsomal RoDH did (42). Unfortunately, this hypothesis is based on ADH4 studies with a spectrophotometric assay having a limit of detection of 2,000 pmol⋅min−1⋅mg−1 (7, 43) and RoDH studies with an HPLC assay with a limit of detection of less than 100 pmol⋅min−1⋅mg−1 (11). Thus, the only conclusion that can be made from these studies is that CRBPI-retinol reduces the activity of ADH4 to some value below 2,000 pmol⋅min−1⋅mg−1, which is significantly lower than its activity with free retinol (<400,000 pmol⋅min−1⋅mg−1), but perhaps still as high or higher than RoDH (115 pmol⋅min−1⋅mg−1). Indeed, CRBPI null mutant mice do not lack RA synthesis, but have excessive RA synthesis and reduced vitamin A storage (44), thus suggesting that CRBPI protects against excessive utilization of retinol by ADHs but does not necessarily reduce activity to zero. Our demonstration that mice need ADH3 and ADH4 to maximize survival and growth during gestational VAD provides evidence that these ADHs can indeed use retinol even when CRBPI-retinol must be the predominant form, and further indicates that SDRs cannot completely fulfill the function of oxidizing retinol to retinal for RA production.
The mammalian ADH gene family was derived from duplications of an ancestral ADH3 gene conserved in lower vertebrate cartilaginous fishes (32) and in chordate invertebrates including Amphioxus (33) and ascidians (45). Because evolutionary data suggest that ADH1 diverged from ADH3 during the advent of bony fishes (32, 46), and that ADH4 diverged from ADH1 during emergence of amphibians (47, 48), ADH1 and ADH4 cannot be responsible for RA synthesis in present-day primitive fishes and their ancestors where retinoid signaling is believed to have originated (1). Instead, our data indicate that ADH3 initially evolved to perform this function, and its relatively low activity with retinol may have actually been advantageous in that it would have spared retinol, particularly important during VAD commonly encountered in the wild, while still making sufficient RA for development. We propose that duplications of an ancestral ADH3 gene have resulted in higher vertebrates obtaining high-activity, tissue-specific forms (ADH1 and ADH4) that function along with low-activity, ubiquitous ADH3 to improve the efficiency of retinoid metabolism for minimization of vitamin A toxicity or deficiency. To support this hypothesis, we demonstrate here that a loss of ADH4 does not result in a significant systemic reduction in metabolism of a toxic dose of retinol, but does result in a significant loss of growth and viability during gestational VAD. Thus, the systemic contribution of ADH4 for oxidation of retinol to RA is relatively low, but evidently localized RA production in ADH4-expressing tissues is very important for growth and survival of higher vertebrates. As for ADH1, the reverse is true, pointing to a role in minimizing retinol toxicity but not VAD in higher vertebrates. ADH3 functions in an intermediate fashion contributing significantly to both systemic retinol clearance and protection against gestational VAD, but not as efficiently as ADH1 or ADH4, respectively. Further support for this hypothesis comes from studies showing that ADH1 and ADH3 are expressed at high levels in liver (as well as ADH3 being ubiquitous) whereas ADH4 is expressed at relatively low levels in peripheral tissues and absent from liver (31, 37, 49). More support comes from studies indicating that Adh1−/− mice exhibit high sensitivity to acute or chronic retinol toxicity, whereas Adh4−/− mice exhibit low sensitivity and Adh3−/− mice exhibit an intermediate level of sensitivity (A.M., X.F., and G.D., unpublished data). Collectively, these findings indicate that low-activity, ubiquitous ADH3 may alone be adequate to catalyze oxidation of retinol to RA under conditions of vitamin A sufficiency, but that it is advantageous to have a high-activity enzyme expressed in peripheral target tissues during VAD (ADH4) and a high-activity enzyme expressed in liver during vitamin A toxicity to increase turnover of excess retinol (ADH1).
We have shown here that ADH4 becomes important during VAD in the mouse suggesting that tissues where ADH4 is expressed (primarily epithelial) may have a greater requirement for RA than other tissues. However, when maintained on a standard diet Adh4−/− mice do not exhibit a growth deficiency as do Adh3−/− mice, indicating that ADH3 is the dominant enzyme for systemic retinal generation. A compound null mutant for Adh3 and Adh4 could be used to examine the overlapping functions of ADH3 and ADH4 during development, but because these two genes are closely linked on the same chromosome this compound null mutant cannot be obtained by simple matings of the single mutants and will instead require more complex methodology.
Higher vertebrate ADHs have also evolved to metabolize a variety of toxic alcohols including ethanol (28). Our findings here should spur further interest in determining whether the mechanisms of alcohol toxicity and teratogenicity involve interactions of alcohol with RA-signaling pathways as hypothesized (50).
Acknowledgments
We thank F. Mic for helpful discussions and H. Freeze and J. Smith for providing access to HPLC systems. This work was supported by National Institutes of Health Grant R01 AA09731 (to G.D.) and by a grant from the Spanish Dirección General de Enseñanza Superior e Investigación Científica (to X.P. and J.F.).
Abbreviations
- ADH
alcohol dehydrogenase
- Adh1
mouse class I ADH gene
- Adh3
mouse class III ADH gene
- Adh4
mouse class IV ADH gene
- RA
retinoic acid
- RALDH2
retinaldehyde dehydrogenase 2
- SDR
short-chain dehydrogenase/reductase
- VAD
vitamin A deficiency
- CRBPI
cellular retinol-binding protein type I
- WT
wild type
Note Added in Proof.
Adh1/Adh4 double null mutant mice were shown to be viable, and it was found that loss of both Adh1 and Adh4does not have additive effects, but instead these genes have distinct functions in vitamin A toxicity and VAD, respectively (51). Also, a new mouse SDR called RDH1 has been reported that is active with retinol and shows more widespread expression than other SDRs (52), although it is unclear whether expression of RDH1 is as widespread as that of ADH3.
References
- 1.Duester G. Eur J Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 2.Kastner P, Mark M, Chambon P. Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 3.Duester G, Farrés J, Felder M R, Holmes R S, Höög J-O, Parés X, Plapp B V, Yin S-J, Jörnvall H. Biochem Pharmacol. 1999;58:389–395. doi: 10.1016/s0006-2952(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Jörnvall H, Höög J O, Persson B. FEBS Lett. 1999;445:261–264. doi: 10.1016/s0014-5793(99)00130-1. [DOI] [PubMed] [Google Scholar]
- 5.Szalai G, Duester G, Friedman R, Jia H, Lin S, Roe B A, Felder MR. Eur J Biochem. 2002;269:224–232. doi: 10.1046/j.0014-2956.2001.02642.x. [DOI] [PubMed] [Google Scholar]
- 6.Boleda M D, Saubi N, Farrés J, Parés X. Arch Biochem Biophys. 1993;307:85–90. doi: 10.1006/abbi.1993.1564. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z-N, Davis G J, Hurley T D, Stone C L, Li T-K, Bosron WF. Alcohol Clin Exp Res. 1994;18:587–591. doi: 10.1111/j.1530-0277.1994.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 8.Han C L, Liao C S, Wu C W, Hwong C L, Lee A R, Yin SJ. Eur J Biochem. 1998;254:25–31. doi: 10.1046/j.1432-1327.1998.2540025.x. [DOI] [PubMed] [Google Scholar]
- 9.Crosas B, Allali-Hassani A, Martínez S E, Martras S, Persson B, Jörnvall H, Parés X, Farrés J. J Biol Chem. 2000;275:25180–25187. doi: 10.1074/jbc.M910040199. [DOI] [PubMed] [Google Scholar]
- 10.Chai X, Boerman M H E M, Zhai Y, Napoli J L. J Biol Chem. 1995;270:3900–3904. doi: 10.1074/jbc.270.8.3900. [DOI] [PubMed] [Google Scholar]
- 11.Boerman M H E M, Napoli J L. Arch Biochem Biophys. 1995;321:434–441. doi: 10.1006/abbi.1995.1415. [DOI] [PubMed] [Google Scholar]
- 12.Chai X, Zhai Y, Popescu G, Napoli J L. J Biol Chem. 1995;270:28408–28412. doi: 10.1074/jbc.270.47.28408. [DOI] [PubMed] [Google Scholar]
- 13.Chai X Y, Zhai Y, Napoli J L. J Biol Chem. 1997;272:33125–33131. doi: 10.1074/jbc.272.52.33125. [DOI] [PubMed] [Google Scholar]
- 14.Su J, Chai X Y, Kahn B, Napoli J L. J Biol Chem. 1998;273:17910–17916. doi: 10.1074/jbc.273.28.17910. [DOI] [PubMed] [Google Scholar]
- 15.Romert A, Tuvendal P, Simon A, Dencker L, Eriksson U. Proc Natl Acad Sci USA. 1998;95:4404–4409. doi: 10.1073/pnas.95.8.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamble M V, Shang E Y, Zott R P, Mertz J R, Wolgemuth D J, Blaner W S. J Lipid Res. 1999;40:2279–2292. [PubMed] [Google Scholar]
- 17.Niederreither K, Subbarayan V, Dollé P, Chambon P. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 18.Mic F A, Haselbeck R J, Cuenca A E, Duester G. Development. U.K.: Cambridge; 2002. , in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederreither K, McCaffery P, Dräger U C, Chambon P, Dollé P. Mech Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 20.Haselbeck R J, Hoffmann I, Duester G. Dev Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mic F A, Molotkov A, Fan X, Cuenca A E, Duester G. Mech Dev. 2000;97:227–230. doi: 10.1016/s0925-4773(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Wagner E, McCaffery P, Smith D, Andreadis A, Dräger U C. Mech Dev. 2000;95:283–289. doi: 10.1016/s0925-4773(00)00352-x. [DOI] [PubMed] [Google Scholar]
- 23.Grün F, Hirose Y, Kawauchi S, Ogura T, Umesono K. J Biol Chem. 2000;275:41210–41218. doi: 10.1074/jbc.M007376200. [DOI] [PubMed] [Google Scholar]
- 24.Ang H L, Deltour L, Hayamizu T F, Zgombic-Knight M, Duester G. J Biol Chem. 1996;271:9526–9534. doi: 10.1074/jbc.271.16.9526. [DOI] [PubMed] [Google Scholar]
- 25.Ang H L, Deltour L, Zgombic-Knight M, Wagner M A, Duester G. Alcohol Clin Exp Res. 1996;20:1050–1064. doi: 10.1111/j.1530-0277.1996.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 26.Ulven S M, Gundersen T E, Weedon M S, Landaas V O, Sakhi A K, Fromm S H, Geronimo B A, Moskaug J O, Blomhoff R. Dev Biol. 2000;220:379–391. doi: 10.1006/dbio.2000.9634. [DOI] [PubMed] [Google Scholar]
- 27.Deltour L, Foglio M H, Duester G. Dev Genet. 1999;25:1–10. doi: 10.1002/(SICI)1520-6408(1999)25:1<1::AID-DVG1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Deltour L, Foglio M H, Duester G. J Biol Chem. 1999;274:16796–16801. doi: 10.1074/jbc.274.24.16796. [DOI] [PubMed] [Google Scholar]
- 29.Driessen CAGG, Winkens H J, Hoffmann K, Kuhlmann L D, Janssen B P M, Van Vugt A H M, Van Hooser J P, Wieringa B E, Deutman A F, Palczewski K, et al. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, Simon A, Eriksson U, Harris E, Berson E L, Dryja T P. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 31.Haselbeck R J, Duester G. Alcohol Clin Exp Res. 1997;21:1484–1490. [PubMed] [Google Scholar]
- 32.Danielsson O, Jörnvall H. Proc Natl Acad Sci USA. 1992;89:9247–9251. doi: 10.1073/pnas.89.19.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cañestro C, Hjelmqvist L, Albalat R, Garcia-Fernàndez J, Gonzàlez-Duarte R, Jörnvall H. Eur J Biochem. 2000;267:6511–6518. doi: 10.1046/j.1432-1327.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 34.Koivusalo M, Baumann M, Uotila L. FEBS Lett. 1989;257:105–109. doi: 10.1016/0014-5793(89)81797-1. [DOI] [PubMed] [Google Scholar]
- 35.Fernández M R, Biosca J A, Norin A, Jörnvall H, Parés X. FEBS Lett. 1995;370:23–26. doi: 10.1016/0014-5793(95)00788-b. [DOI] [PubMed] [Google Scholar]
- 36.Collins M D, Eckhoff C, Chahoud I, Bochert G, Nau H. Arch Toxicol. 1992;66:652–659. doi: 10.1007/BF01981505. [DOI] [PubMed] [Google Scholar]
- 37.Algar E M, Seeley T-L, Holmes R S. Eur J Biochem. 1983;137:139–147. doi: 10.1111/j.1432-1033.1983.tb07807.x. [DOI] [PubMed] [Google Scholar]
- 38.Von Lintig J, Vogt K. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 39.Deltour L, Haselbeck R J, Ang H L, Duester G. Biol Reprod. 1997;56:102–109. doi: 10.1095/biolreprod56.1.102. [DOI] [PubMed] [Google Scholar]
- 40.Haselbeck R J, Ang H L, Duester G. Dev Dyn. 1997;208:447–453. doi: 10.1002/(SICI)1097-0177(199704)208:4<447::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Haselbeck R J, Ang H L, Deltour L, Duester G. Endocrinology. 1997;138:3035–3041. doi: 10.1210/endo.138.7.5274. [DOI] [PubMed] [Google Scholar]
- 42.Napoli J L. Biochim Biophys Acta Mol Cell Biol Lipids. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 43.Kedishvili N Y, Gough W H, Davis W I, Parsons S, Li T K, Bosron W F. Biochem Biophys Res Commun. 1998;249:191–196. doi: 10.1006/bbrc.1998.9105. [DOI] [PubMed] [Google Scholar]
- 44.Ghyselinck N B, Båvik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson C B, Håkansson H, Sauvant P, et al. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cañestro C, Albalat R, Hjelmqvist L, Godoy L, Jörnvall H, González-Duarte R. J Mol Evol. 2002;54:81–89. doi: 10.1007/s00239-001-0020-2. [DOI] [PubMed] [Google Scholar]
- 46.Danielsson O, Shafqat J, Estonius M, Jörnvall H. Eur J Biochem. 1994;225:1081–1088. doi: 10.1111/j.1432-1033.1994.1081b.x. [DOI] [PubMed] [Google Scholar]
- 47.Parés X, Cederlund E, Moreno A, Hjelmqvist L, Farrés J, Jörnvall H. Proc Natl Acad Sci USA. 1994;91:1893–1897. doi: 10.1073/pnas.91.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann I, Ang H L, Duester G. Dev Dyn. 1998;213:261–270. doi: 10.1002/(SICI)1097-0177(199811)213:3<261::AID-AJA3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 49.Zgombic-Knight M, Ang H L, Foglio M H, Duester G. J Biol Chem. 1995;270:10868–10877. doi: 10.1074/jbc.270.18.10868. [DOI] [PubMed] [Google Scholar]
- 50.Deltour L, Ang H L, Duester G. FASEB J. 1996;10:1050–1057. [PubMed] [Google Scholar]
- 51. Molotkov, A., Deltour, L., Foglio, M. H., Cuenca, A. E. & Duester, G. (2002) J. Biol. Chem., in press. [DOI] [PMC free article] [PubMed]
- 52.Zhang M, Chen W, Smith S M, Napoli J L. J Biol Chem. 2001;276:44083–44090. doi: 10.1074/jbc.M105748200. [DOI] [PubMed] [Google Scholar]