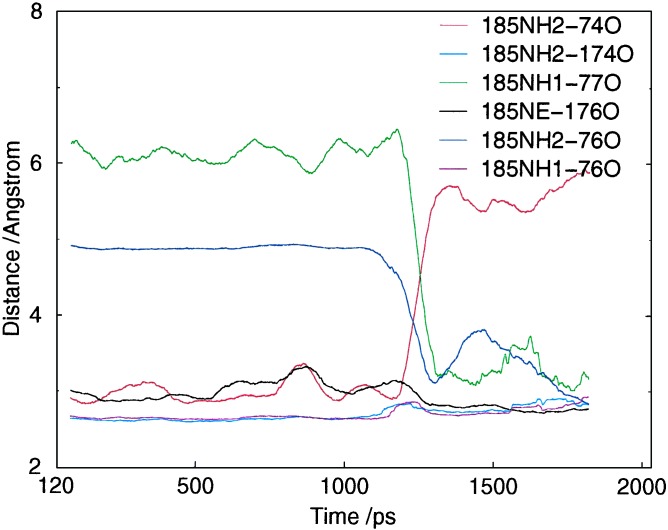
Figure 5.
Interchange of hydrogen bonds to R185 in P450eryf, as observed during simulation MD-SOLV. The hydrophobic environment around R185 restricts its solvent interactions for over 1 ns. However solvent interactions with R185 do become more frequent, and eventually lead to an external water molecule penetrating through to the active site. During this penetration process there is breakage/rearrangement of the hydrogen bond interactions of R185. This process can be seen in the figure to occur between 1.1 and 1.3 ns. The overall change of structure by the end of the simulation is one where R185 has moved slightly toward the bulk solvent, has a greater interaction with the bulk solvent through NH1, and has slightly weaker hydrogen bonding to the B/B′ loop. This is similar to the sorts of changes seen in REMD and may indicate how channel opening could start in the unliganded protein (Fig. 8 contains a similar plot for a REMD trajectory).