Abstract
Recent work on wild birds has revealed the importance of sperm competition as a source of sexual selection, but behavioral and paternity studies have previously provided only indirect evidence for mechanisms of sperm competition in wild birds. In a field study of collared flycatchers Ficedula albicollis we used a previously uncharacterized method to determine the frequency and timing of extra-pair inseminations. By counting the number of sperm trapped on the perivitelline layer of eggs, we determined the timing of inseminations and estimated, on a day-to-day basis, the amount of sperm females stored. Our results showed that female collared flycatchers preferentially engaged in extra-pair copulations when mated to an unattractive male with a small white forehead patch. These copulations were timed for the middle part of their fertile period, at least 2 days after the last within-pair insemination. Although the mean number of extra-pair insemination events was only 1.33 per cuckolding female, the ratio between the number of sperm from extra-pair and pair inseminations was at least 5 to 1. Thus a single, well timed extra-pair insemination caused by female behavior could greatly bias fertilization probability in favor of an attractive extra-pair male. Our results suggest a possible behavioral mechanism for female control of sperm competition.
Numerous studies of birds have shown that females copulate with more than one male during their fertile period (1–3). As a result, sperm from different males may compete to fertilize a single clutch of eggs (1–3). The increased variance in male reproductive success resulting from extra-pair fertilizations is the most important source of sexual selection in socially monogamous bird species (4). The temporal pattern of copulation in socially monogamous birds typically has an early peak with a subsequent decrease before the eggs are laid (5, 6). Several studies show that the probability that females engage in extra-pair copulations (EPCs) depends on the phenotypic quality of their mate. Females mated to males of low quality often engage in one or more EPC with a preferred male, whereas females paired to high-quality males usually copulate only with their mates (7–9). EPCs seem generally to be rare, but extra-pair fertilizations may be much more common than expected, given observed rates of EPC (10–15). One possible reason for the difference is that females may time EPCs to coincide with peak fertility (16). Alternatively, EPCs may be more difficult to observe, and from field observations it is difficult to determine whether a copulation was successful. These possibilities can be distinguished if field data can tell whether insemination has occurred. Here, we describe a method for distinguishing these possibilities, and present results obtained by using it on a population of collared flycatchers Ficedula albicollis.
Extensive work on sexual selection in wild populations of collared flycatchers has shown that females prefer males with large forehead patches (17), and that such males attract multiple females more frequently than males with small forehead patches (18). The forehead patch is a heritable condition-dependent secondary sexual character (18–20) affecting male success in sperm competition. Males with smaller forehead patches lose more paternity because of EPCs (21, 22), and they tend to lose paternity to males with larger forehead patches (21, 22). Offspring of large patched males are in better condition at fledging than offspring of males with a small patch (20). Thus, females may use the size of the forehead patch of their mate as a cue for pursuing EPCs.
The method we developed to study sperm competition in the wild involved attaching a rubber ring around the cloaca of randomly chosen males. This ring had a height that made sperm transfer impossible, but still allowed males to ejaculate and defecate. Experimental males were thus prevented from transferring sperm during copulations with their mates for a period from 2 days before to 3 days after the start of laying (henceforth termed the main fertile period). In these experimental pairs, females that were prevented from being inseminated by their mate could be inseminated only by EPCs. In control pairs, females could be inseminated by both pair and extra-pair males. Key determinants of the outcome of sperm competition are the timing of pair and extra-pair copulations, the proportion of sperm transferred by the pair and the extra-pair male (23–25), and relative sperm quality (26). We estimated these variables by counting sperm on the inner perivitelline layer of eggs laid by females in the two groups (27, 28).
Methods
Study Population and Collection of Eggs.
We worked in the Pilis Hills, Hungary (47°42′ N, 19°01′ E), a Central European oak forest that is a typical habitat for the collared flycatcher. This small, sexually dichromatic, migratory passerine species nests in artificial nest boxes in our plots. The modal clutch size is 6 eggs. Only the female incubates and broods the young, although the male brings some food to growing nestlings, and there are sex differences in risk-taking with respect to experimental presentations of predators (29). We gathered data from 15 experimental and 18 control nests. Eggs were numbered by using an indelible marker on the day that they were laid until a total of six eggs had been collected from each clutch. Eggs were stored in a refrigerator (at +5°C) for 3–4 days before examination.
Experimental Procedures.
Three days after pair formation, 1–3 days before egg laying, we captured experimental males and attached the rubber ring (an “anticopulator”) around the cloaca of the male, thereby preventing him from transferring sperm during copulations. We attached the ring by gluing it to the skin around the base of the cloaca, reinforced with three narrow cloth ribbons (1–2 cm in length), which were glued to the ring and to the surrounding plumage. We checked the rubber ring each day by using a telescope; all rings remained in their original position, and all pair bonds remained intact throughout the experiment. Males wore their rings until completion of the clutch; 11 males were recaptured, and their rings were removed 1–3 days after the onset of incubation; 4 males could not be recaptured. We detected no sign of unusual behavior (e.g., pecking directed at the ring, other stress-related activities) during our observations. Females mated to males with rubber rings could receive sperm only from extra-pair males beginning 2 days before the start of laying. All inseminations after this time were from EPCs.
We measured the height and width of the forehead patch (a character shown to be important in sperm competition; refs 20–22) of the males we trapped with calipers to the nearest 0.1 mm. The size of the forehead patch was estimated from the product of maximum width and height. The median of the forehead patch of 15 experimental males with rubber rings was 73 mm2 (range: 49–85 mm2), which did not differ from the control group (median: 66 mm2, range: 55–92 mm2, Mann–Whitney U test, z = 1.050, P = 0.19).
Efficacy of Anticopulator.
We performed two experiments to test the efficacy of the anticopulator, one under controlled laboratory conditions, and one in the field. Experiment 1 used 10 individually caged pairs of zebra finches Taeniopygia guttata, a passerine bird of similar size to the collared flycatcher. It was designed to test the efficacy of the anticopulator when females could copulate only with their mates. In five randomly selected pairs, the males were fitted with an anticopulator 8–19 days before the laying of the first egg. In the other five pairs, males received no anticopulator, so copulation was ad libitum. Eggs were collected as they were laid, replaced with dummy eggs, and stored in a refrigerator before having sperm numbers counted, as described below.
Experiment 2 performed in May–June 2001 at our field study site was designed to test the effect of the anticopulator on rates of extra-pair paternity in the field. We captured nine males after pairing [1–7 days (mean 3.7 ± 0.7 SE days) before the first egg of the clutch was laid] and fitted them with anticopulators. Three of these males could not be relocated during incubation, and the clutches were collected after they had been incubated for at least 3 days (approximately 25% of the normal incubation period). In two of these clutches, embryos had partially developed and were genotyped; in the third clutch, all seven eggs failed to develop any visible embryo and were apparently infertile. We collected blood samples from nestlings in the other six nests and stored them in buffer. In addition, we collected blood samples from both parents (from the male when the experiment was performed and from the female during incubation or nestling feeding). Paternity was determined on the basis of allele-sharing at three polymorphic microsatellite loci, FhU2, FhU3, and FhU4 as previously described (21, 30, 31). In these previous studies (of Swedish and Czech collared flycatcher populations), the combined exclusion power of these markers was 0.96, and offspring that mismatched their social father at one or more loci were considered to have been fathered by an extra-pair fertilization. Although the relatively low exclusion power suggests that some cases of extra-pair paternity may not have been detected, we compare the estimate derived here with others derived by using the same set of markers. Hence, the error should be similar in each case. The mutation rate of these loci is low: no mutations have been detected in over 500 meiotic events observed between mothers and their offspring in our previous studies.
Sperm Counts.
We counted the number of sperm trapped on the inner perivitelline layer of eggs by using a standard technique (26). We stained the nuclei of spermatozoa with fluorescent Hoescht dye 33342 (Sigma), and counted them by using a Leica microscope with Leitz 2000 lens at 200× and 400× magnification. The number of spermatozoa on the perivitelline layer is correlated with the number of sperm present in the female genital tract, but also provides the best estimate of the overall number of sperm reaching the ovum at the site of fertilization (26, 27).
Dating Insemination Events.
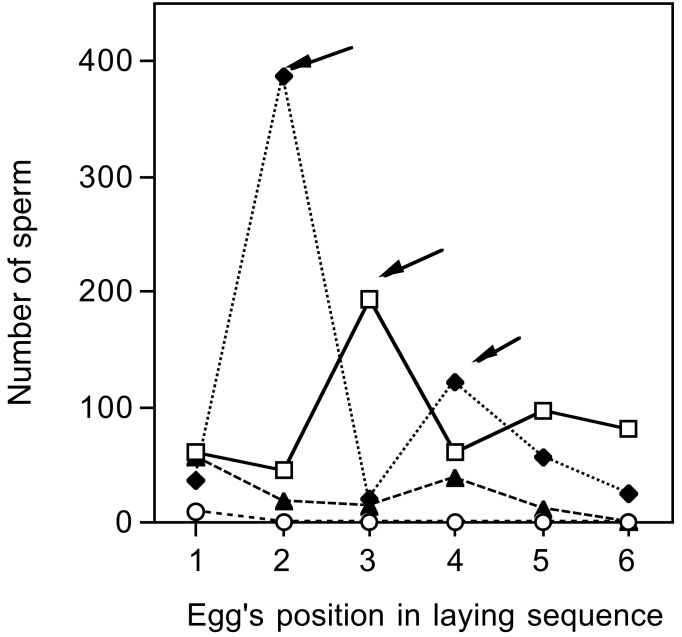
In nonpasserine birds, sperm reach the site of fertilization 48 h after insemination (26). Single insemination events can be detected as a sharp increase in the number of sperm on the inner perivitelline layer of eggs laid on consecutive days. We dated insemination events based on peaks in the number of sperm, assuming a 48-h interval between insemination and when sperm reach the site of fertilization. This assumption may be incorrect, because nothing is known about the duration of this interval in passerines, but any error simply shifts the relative position of all inseminations equally. The peaks were generally sharp; the increase in the number of sperm was limited to a single day (Fig. 1). We cannot distinguish between single and multiple copulations on the same day, so we cannot estimate the number of copulations from the number of inseminations. If peaks lasted 2–3 days, we concluded that insemination events had occurred on consecutive days.
Figure 1.
Examples from four experimental clutches of the numbers of sperm on the inner perivitelline layer of successive eggs within the clutch. In this case, two clutches are those for which there was no copulation after the paired male was prevented from inseminating the female, whereas the other two clutches show a single insemination event inferred to have occurred on day 0 (from sperm counted on the third egg of the clutch; arrow), and two insemination events inferred to have occurred on days −1 and +1 (from sperm counted on the second and fourth eggs of the clutch, respectively; arrow).
Inseminations during different parts of the fertile period may differ in fertilization probability, especially if there is sperm competition (24, 25). An early insemination can potentially fertilize an entire clutch, but the probability of fertilization declines during the laying sequence because of passive sperm loss. Any subsequent copulation with another male may thus decrease the fertilization probability from prior insemination because of last-male sperm precedence. Late pair inseminations can fertilize only the remaining eggs, but there is a reduced probability that extra-pair inseminations at that time will reduce overall paternity. Therefore, we divided the main fertile period covered by our experiment into three 2-day periods: early (−2, −1 days), middle (0, +1 days), and late (+2, +3 days) phases, where the first egg of the clutch is laid on day 0.
Results
Efficacy of Anticopulator.
Experiment 1.
The mean number of sperm on the inner perivitelline layer of eggs produced by experimental zebra finch pairs (3.6 ± 1.5 SE) was much less than in controls (91.4 ± 11.8 SE; t = 7.36, df = 8, P < 0.001). Of the five experimental pairs, no sperm were found in any of the eggs laid by two females; sperm were found only in the first egg of the clutch in the other three pairs, suggesting that the small number of sperm present on the inner perivitelline layer may have resulted from copulations before the anticopulator was fitted (pairs were already formed when the experiment was initiated). Hence, the anticopulator was effective in preventing sperm transfer from male to female under controlled conditions in this species.
Experiment 2.
Nine clutches, comprising a total of 55 eggs, were laid by females paired to males wearing the anticopulatory device. One entire clutch was apparently infertile, as no embryonic development was apparent after 3 days of incubation. In the remaining eight clutches, seven (87.5%) contained nestlings sired by a male other than the one paired to their mother. Two of the eight males fathered none of the nestlings; neither male was one that had disappeared early in incubation. Three previous studies of paternity in unmanipulated pairs of collared flycatchers have all found rather similar rates of extra-pair paternity at the family level: 26/79 (32.9%), 6/14 (42.9%), and 10/29 (34.5%) of broods contained extra-pair young (populations and year of sampling: Sweden 1994 in ref. 21, Sweden 2000 in ref. 31, and Czech Republic in ref. 31, respectively). The proportion of broods containing extra-pair young was significantly higher in the experimental birds than in any of these three samples (G test, G1 ≥ 4.62, P < 0.032); the three nonexperimental samples did not differ in their rate of extra-pair paternity (G2 = 0.51, P = 0.78).
Of 48 offspring genotyped, 30 (63.3%) were not sired by the male paired to the mother of the offspring. This is a significant departure from the levels of extra-pair fertilizations found naturally in Sweden in two different years: 71/459 (15.5%) and 14/81 (17.3%) (refs. 21 and 31, respectively) and the Czech Republic; 21/158 offspring (13.3%) (21) (Generalized Linear Model, binomial errors, correcting for overdispersion: F3,122 = 4.51, P = 0.0049). Thus, in pairs in which the male wore the anticopulator, approximately four times as many offspring were sired by EPCs as in control pairs. The correlation between the time that the male wore the anticopulator and the proportion of the brood sired by extra-pair fertilizations was not statistically significant, perhaps reflecting the small sample size (rS = 0.54, n = 8, P = 0.17). On the basis of the paternity analysis, we conclude that the anticopulator is effective in preventing insemination by free-living male collared flycatchers. Although experimental males gained some paternity (36.6% of nestlings on average), we assume that they resulted from copulations occurring after pair formation, but before application of the anticopulator.
Effect of Treatment on Sperm Numbers.
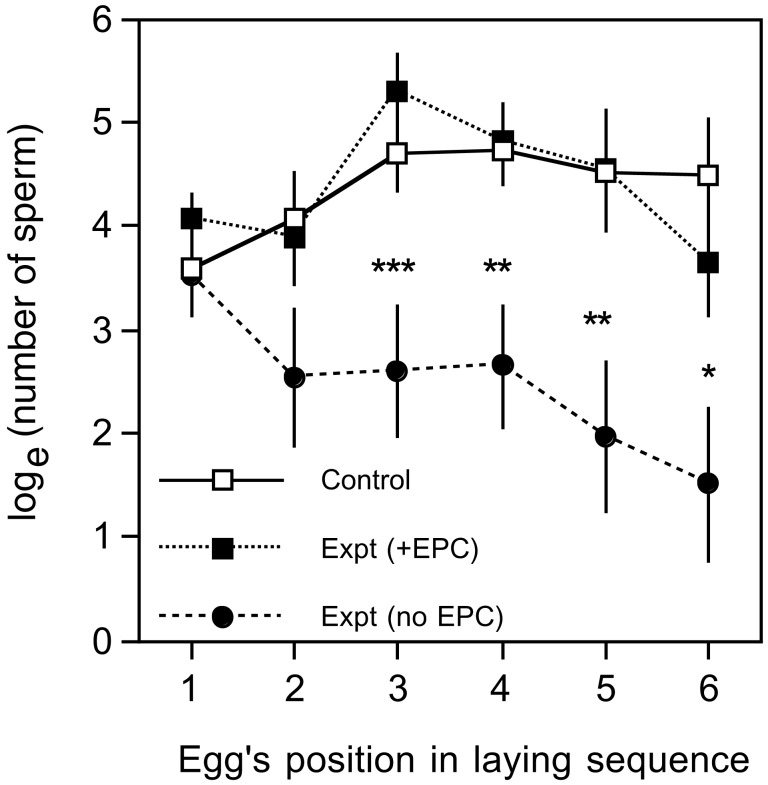
The eggs collected from clutches of experimental and control females indicate that experimental females fell into two groups. In 9/15 females (60%), the female apparently did not receive sperm during the experiment, because the numbers of sperm on the perivitelline layer of successively laid eggs gradually declined, with no sudden increases in numbers of sperm (Fig. 2). In the remaining six females (40%), sperm numbers suddenly increased on one or more of the eggs of the clutch, a pattern seen in all of the 18 control clutches (Fig. 2). Sperm numbers on the eggs of the females that apparently did not copulate differed significantly both from the experimental females that did copulate, and the control females (Fig. 2), after the first egg of the clutch was laid. We interpret these differences as indicating that 40% of the experimental females had received extra-pair inseminations from one or more males other than the one to which they were paired. This frequency of extra-pair insemination is similar to the frequency of broods of collared flycatchers containing extra-pair offspring (33–43%) in Swedish and Czech populations (21, 31). In the experimental clutches where extra-pair inseminations had not occurred, we estimated the instantaneous rate of sperm loss from the sperm storage tubules (23) as 0.019 loge (N spermatozoa) per h (SE = 0.008). This rate is among the lowest recorded among the monogamous passerines (mean of seven other species = 0.030: refs 32–34).
Figure 2.
Mean (± SE) loge (number of spermatozoa on inner perivitelline layer) of successive eggs within the clutch for three groups of females (control females, experimental females that received extra-pair inseminations and experimental females that received no extra-pair inseminations). Asterisks between points indicate the results of Tukey post hoc tests comparing means for the two groups of experimental females (*, P < 0.05; **, P < 0.01; ***, P < 0.001); no other between group comparisons indicated a statistically significant difference.
Timing of Inseminations.
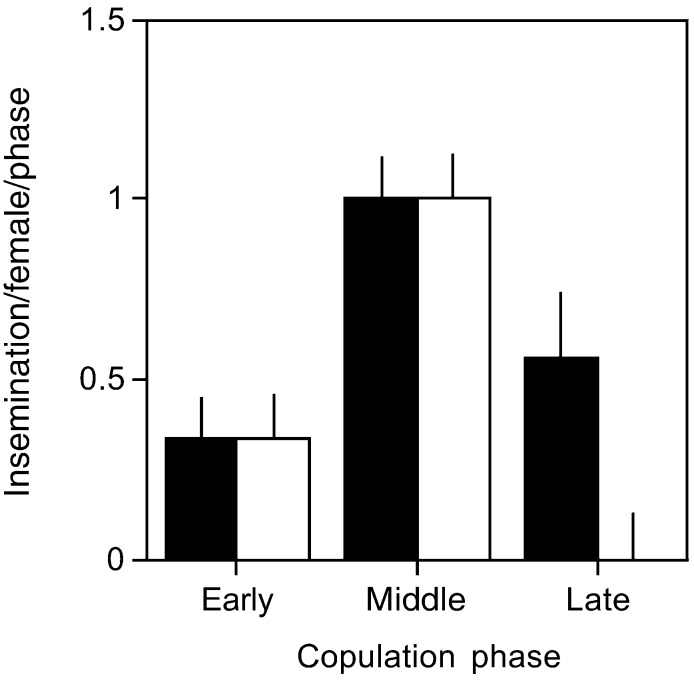
We estimated the frequency of inseminations from the number of peaks in sperm numbers in the clutches of control females. This observation revealed that the frequency of inseminations varied with the stage of the fertile period (repeated measures ANOVA F2,34 = 5.95, P = 0.006; Fig. 3). Inseminations peaked during the middle phase of the fertile period (Scheffé post hoc tests: phase 1 vs. phase 2: P < 0.05; phase 2 vs. phase 3: P < 0.10; Fig. 3). Mean numbers of sperm on the perivitelline layer (93, 143, and 113 for the three phases, respectively) matched the pattern for copulation frequency [ordered heterogeneity test (ref. 35) based on repeated-measures ANOVA; rSPc = 0.816, k = 3, P < 0.05]. These data suggest that female collared flycatchers typically reduce their copulation frequency for a 2-day period just before laying their first egg. Extra-pair inseminations showed a similar pattern, with a peak during the middle phase of the fertile period, suggesting that females may be selectively timing EPCs to this period (repeated-measures ANOVA F2,10 = 17.50, P = 0.0005; Scheffé post hoc tests: phase 1 vs. phase 2: P < 0.05; phase 2 vs. phase 3: P < 0.05 Fig. 3). Unlike within-pair copulations for the control females, there was no evidence that EPCs occurred during the late period of the fertile period. Our technique suggested that EPCs were relatively infrequent: of the six females that engaged in EPCs, four did so on only 1 day; the remaining two did so on 2 different days. We discuss the implications of infrequent insemination for potential mechanisms of female control of sperm competition below.
Figure 3.
Temporal distribution of the frequency of all inseminations for control pairs (black bars) and extra-pair inseminations in the experimental pairs (white bars). The frequency of inseminations had a heterogeneous distribution in both groups. Insemination events were less frequent in the early phase than in the subsequent phase. In experimental pairs, all inseminations were extra-pair inseminations because copulations with the pair male were prevented.
Characteristics of Cuckolded Males.
If the pursuit of EPCs is a female-driven strategy, used to achieve inseminations by males of higher phenotypic quality than their current social mate, mates of experimental females that engaged in EPCs should differ from those that did not. This was indeed the case: females that engaged in EPCs were mated to males with smaller forehead patches than females that did not engage in EPCs (logistic regression: χ21 = 4.530, P = 0.033). Six of eight females mated to males with a forehead patch smaller than median engaged in EPCs. Seven females that had mates with a forehead patch larger than median did not engage in EPCs during 36 fertile days (Fisher exact test, P = 0.007). This finding corroborates work on a Swedish population of collared flycatchers that indicates an important role for forehead patch size in success at sperm competition (21, 22).
Implications for Female Control of Sperm Competition.
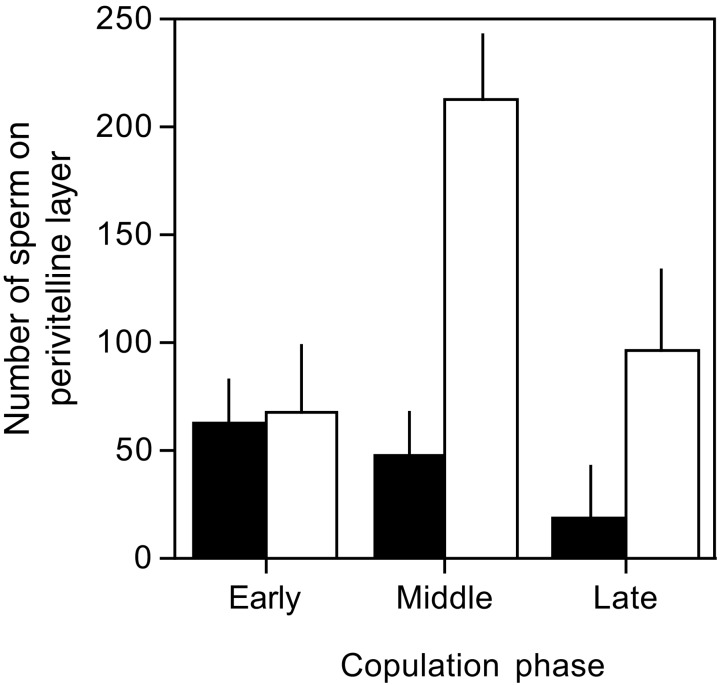
Our results suggest that both pair and EPCs are infrequent in collared flycatchers in the period from 2 days before to 3 days after the start of egg-laying. The temporal distribution of inseminations, and the number of sperm stored by the female, allow inferences to be drawn about the likely outcomes of sperm competition in this species. The attachment of the rubber ring to experimental males, which prevented insemination, mimics the situation where a cuckolding female rejects copulations with her own mate. We can use the number of sperm present in the early insemination phase, and the empirically calculated rate of sperm loss, to calculate the number of sperm present during the later phases of copulation for a female that rejects copulations with her mate, and compare this number with the number of sperm present because of extra-pair inseminations (derived from the experimental females which engaged in EPCs). This calculation shows (Fig. 4) that only 16–18% of sperm present during the middle and late phases of insemination came from the pair male. Hence, by rejecting copulation with the her mate, and engaging in a single EPC with a preferred male during this period, a female would be able to increase the chance that the extra-pair male's sperm will fertilize the eggs, even if the per sperm chances of fertilization are similar for the two males (6).
Figure 4.
Relative numbers of pair and extra-pair male spermatozoa when a female rejects copulations with her own mate during the middle and late phases. Open bars show mean sperm numbers (+SE) from eggs laid by experimental females that cuckolded their mates. Filled bars show mean numbers (+SE) of sperm present on the eggs of experimental females that did not have EPCs, and assumed to all originate from the within-pair male.
Discussion
By experimentally preventing paired males from inseminating their mates, and counting the numbers of sperm trapped around the eggs that the females laid, we were able to show that 40% of females engaged in EPCs between 2 days before and 3 days after clutch initiation, and that they were more likely to do so if paired to unattractive mates. Our data suggest that females would be able to increase the probability of having eggs fertilized by preferred extra-pair mates by controlling the timing of inseminations. They therefore offer a potential mechanistic explanation for the increased success of preferred individuals in sperm competition (see also ref. 37). We discuss alternatives to female control of sperm competition below.
Observations of EPCs in other species (7, 38), and the fact that female collared flycatchers may gain genetic benefits from extra-pair fertilizations, suggest that females may both gain from, and have the opportunity to control, sperm competition (20, 21). Our finding that females in experimental pairs mated to large-patched males were not inseminated by extra-pair males might indicate that females can control sperm competition, although another interpretation is possible. The outcome of male–male competition for a nest hole among collared flycatchers is partially determined by the size of the forehead patch of competing males, with larger patched males being behaviorally dominant (39). A male with a large forehead patch might thus be more effective at guarding his mate than a male with small forehead patch.
Distinguishing these hypotheses requires careful collection of detailed behavioral data, although the apparent low frequency of inseminations suggested by our data make this particularly challenging. However, a previous study of sperm competition in collared flycatchers (22) found evidence of some female control over mating decisions. In a male replacement experiment, where competition between pair and extra-pair males was excluded, pair males with large forehead patches were less likely to be replaced and they fathered a greater proportion of the brood than did males with small patches.
The low copulation rate in collared flycatchers implied by our study, and the period just before egg-laying with very few copulations, may provide females with opportunities to modify the paternity of their brood. If the female were first to copulate with her mate several times, last male sperm precedence might then arise because the share of paternity obtained by the second male increases the greater the interval between inseminations by the first and second male (23, 24). In the collared flycatcher, inseminations by pair and extra-pair males may be temporally separated by a period of low copulation intensity, which would substantially increase the fertilization probability of the last male to mate (23, 40). Our data suggest that if females do not copulate with their mate (as was the case for our experimental females) during the middle and late phases, sperm from extra-pair inseminations will have a very high probability of fertilizing the remaining three eggs of the clutch. However, our control data (Fig. 3) show that in nonmanipulated pairs there are probably pair inseminations in the late phase, which may reduce the probability of extra-pair fertilization for the later-ovulated eggs. Females face a tradeoff between the number of young sired by EPCs and the amount of parental assistance they receive from their mate, because males reduce parental care when their certainty of paternity is reduced (36, 41). Our results suggest that, on average, one would expect two or three extra-pair young in a cuckolding collared flycatcher female's brood, which coincides well with paternity data from a Swedish population (21). In addition, the proportion of females engaging in EPCs agrees well with the proportion of females having extra-pair young within their broods in the same population (21).
Female collared flycatchers seem to base their copulation decisions on the relative attractiveness of their mate, and preferentially copulate with an extra-pair male when mated to an unattractive male (21, 22). If a cuckolding female were to refrain from inseminations with her mate for two days before any extra-pair inseminations, even a single extra-pair event could have dramatic consequences for paternity because of the timing of copulation in combination with passive sperm loss. Our results thus suggest a simple behavioral mechanism with potential to explain the commonly encountered discrepancy between the proportion of copulations that are EPCs and the proportion of offspring which are extra-pair offspring, and suggest that females would be able to achieve a substantial degree of control through the timing of these occasional events.
Acknowledgments
We thank T. Birkhead, A. P. Møller, G. J. Wishart, V. Altbäcker, Á. Szentesi, and two referees for comments on the manuscript, and J. Barna for counting sperm. Collection of eggs was made under license of the Authority for Nature Conservation. This study was supported by grants from Hungarian National Science Foundation (Országos Tudományos Kutatási Alapprogramok) and the Hungarian Ministry for Education, and by fellowships from Natural Environment Research Council (U.K.) and the Royal Society (to S.C.G. and B.C.S.).
Abbreviation
- EPC
extra-pair copulation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Birkhead T R, Møller A P. Sperm Competition in Birds. London: Academic; 1992. [Google Scholar]
- 2.Birkhead T R, Møller A P. Sperm Competition and Sexual Selection. London: Academic; 1998. [Google Scholar]
- 3.Westneat D F, Sherman P W. Behav Ecol Sociobiol. 1997;41:205–215. [Google Scholar]
- 4.Møller A P, Ninni P. Behav Ecol Sociobiol. 1998;43:345–358. [Google Scholar]
- 5.Birkhead T R, Atkin L, Møller A P. Behaviour. 1987;101:101–138. [Google Scholar]
- 6.Birkhead T R, Møller A P. Anim Behav. 1993;45:105–118. [Google Scholar]
- 7.Kempenaers B, Verheyen G R, van den Broeck M, Burke T, van Broeckhoven C, Dhondt A A. Nature (London) 1992;357:494–496. [Google Scholar]
- 8.Sundberg J, Dixon A. Anim Behav. 1996;52:113–122. [Google Scholar]
- 9.Hasselquist D, Bensch S, von Schantz T. Nature (London) 1996;381:229–232. [Google Scholar]
- 10.Lifjeld J T, Dunn P O, Robertson R J, Boag P T. Anim Behav. 1993;45:213–229. [Google Scholar]
- 11.Perrault S, Lemon R E, Kuhnlein U. Behav Ecol. 1998;8:612–621. [Google Scholar]
- 12.Mulder R A, Dunn P O, Cockburn A, Lazenby-Cohen K A, Howell M J. Proc R Soc London B. 1994;255:223–229. [Google Scholar]
- 13.Dixon A, Ross D, O'Malley S L C, Burke T. Nature (London) 1994;371:698–700. [Google Scholar]
- 14.Dunn P O, Lifjeld J T. Anim Behav. 1994;47:983–985. [Google Scholar]
- 15.Birkhead T R, Møller A P. Anim Behav. 1995;49:843–848. [Google Scholar]
- 16.Eberhard WG. Female Control. Princeton: Princeton Univ. Press; 1996. [Google Scholar]
- 17.Qvarnström A, Pärt T, Sheldon B C. Nature (London) 2000;405:344–347. doi: 10.1038/35012605. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson L, Qvarnström A, Sheldon B C. Nature (London) 1995;375:311–313. [Google Scholar]
- 19.Ellegren H, Gustafsson L, Sheldon B C. Proc Natl Acad Sci USA. 1996;92:11723–11728. doi: 10.1073/pnas.93.21.11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheldon B C, Merilä J, Qvarnström A, Gustafsson L, Ellegren H. Proc R Soc London B. 1997;264:297–302. [Google Scholar]
- 21.Sheldon B C, Ellegren H. Anim Behav. 1999;57:285–298. doi: 10.1006/anbe.1998.0968. [DOI] [PubMed] [Google Scholar]
- 22.Sheldon B C, Davidson P, Lindgren G. Behav Ecol Sociobiol. 1999;46:141–148. [Google Scholar]
- 23.Birkhead T R, Biggins J D. Behav Ecol. 1998;9:253–260. [Google Scholar]
- 24.Colegrave N, Birkhead T R, Lessells C M. Proc R Soc London B. 1995;259:223–228. [Google Scholar]
- 25.Birkhead T R, Fletcher F, Pellatt E J, Staples A. Nature (London) 1995;377:422–423. [Google Scholar]
- 26.Birkhead T R, Martinez J G, Burke T, Froman D P. Proc R Soc London B. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wishart G J. J Reprod Fert. 1987;80:493–498. doi: 10.1530/jrf.0.0800493. [DOI] [PubMed] [Google Scholar]
- 28.Brillard J P, Bakst M R. Biol Reprod. 1990;43:271–275. doi: 10.1095/biolreprod43.2.271. [DOI] [PubMed] [Google Scholar]
- 29.Michl G, Török J, Garamszegi L Z, Tóth L. Anim Behav. 2000;59:623–628. doi: 10.1006/anbe.1999.1352. [DOI] [PubMed] [Google Scholar]
- 30.Sheldon B C, Ellegren H. Proc R Soc London B. 1996;263:1017–1021. [Google Scholar]
- 31.Veen T, Borge T, Griffith S C, Sætre G-P, Bures S, Gustafsson L, Sheldon B C. Nature (London) 2001;411:45–50. doi: 10.1038/35075000. [DOI] [PubMed] [Google Scholar]
- 32.Birkhead T R, Sheldon B C, Fletcher F. J Reprod Fert. 1994;101:353–361. doi: 10.1530/jrf.0.1010353. [DOI] [PubMed] [Google Scholar]
- 33.Sax A, Hoi H, Birkhead T R. Anim Behav. 1998;56:1199–1204. doi: 10.1006/anbe.1998.0859. [DOI] [PubMed] [Google Scholar]
- 34.Birkhead T R, Fletcher F J. J Reprod Fert. 1998;114:141–145. doi: 10.1530/jrf.0.1140141. [DOI] [PubMed] [Google Scholar]
- 35.Rice W R, Gaines S D. Proc Natl Acad Sci USA. 1994;91:225–226. doi: 10.1073/pnas.91.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheldon B C, Ellegren H. Proc R Soc London B. 1998;265:1737–1742. [Google Scholar]
- 37.Pizarri T, Birkhead T R. Nature (London) 2000;405:787–789. doi: 10.1038/35015558. [DOI] [PubMed] [Google Scholar]
- 38.Sheldon B C. Anim Behav. 1994;47:163–173. [Google Scholar]
- 39.Pärt T, Qvarnström A. Anim Behav. 1997;54:893–899. [Google Scholar]
- 40.Birkhead T R, Wishart G J, Biggins J D. Proc R Soc London B. 1995;261:285–292. [Google Scholar]
- 41.Sheldon B C, Räsänen K, Dias P C. Behav Ecol. 1997;8:421–428. [Google Scholar]