Abstract
Atrazine is the most commonly used herbicide in the U.S. and probably the world. It can be present at several parts per million in agricultural runoff and can reach 40 parts per billion (ppb) in precipitation. We examined the effects of atrazine on sexual development in African clawed frogs (Xenopus laevis). Larvae were exposed to atrazine (0.01–200 ppb) by immersion throughout larval development, and we examined gonadal histology and laryngeal size at metamorphosis. Atrazine (≥0.1 ppb) induced hermaphroditism and demasculinized the larynges of exposed males (≥1.0 ppb). In addition, we examined plasma testosterone levels in sexually mature males. Male X. laevis suffered a 10-fold decrease in testosterone levels when exposed to 25 ppb atrazine. We hypothesize that atrazine induces aromatase and promotes the conversion of testosterone to estrogen. This disruption in steroidogenesis likely explains the demasculinization of the male larynx and the production of hermaphrodites. The effective levels reported in the current study are realistic exposures that suggest that other amphibian species exposed to atrazine in the wild could be at risk of impaired sexual development. This widespread compound and other environmental endocrine disruptors may be a factor in global amphibian declines.
In the last 10 years, a great deal of attention has focused on the global presence of endocrine-disrupting contaminants in the environment (1, 2). Similarly, a great deal of attention has focused on global amphibian declines (3, 4). In the case of amphibian declines, efforts focus on identifying causes (5), whereas for endocrine disruptors, the “causes” have been identified and studies focus on identifying effects of endocrine disruptors in the environment (6–11).
Atrazine (2-chloro-4-ethytlamino-6-isopropylamine-1,3,5-triazine) is the most commonly used herbicide in the U.S. and probably the world. The U.S. Department of Agriculture reports that more than 30,000 tons (60 million pounds) are used annually in the U.S. alone (12). Atrazine has been used for over 40 years and currently it is used in more than 80 countries. Despite its widespread intensive use, atrazine is considered safe because of its short half-life and negligible bioaccumulation and biomagnification (13). Also, atrazine seems to have very few effects on adults and reportedly induces abnormalities and deformities only at very high doses. As a result of the high doses required to produce deformities, it has been suggested that “direct toxicity of atrazine is probably not a significant factor in recent amphibian declines” (14). Here, we test the hypothesis that atrazine may interfere with metamorphosis and sex differentiation at ecologically relevant low doses via endocrine-disrupting mechanisms.
Materials and Methods
Animal Breeding and Larval Care.
We report results from two experiments that used frogs from two separate sources. Adults from Exp. 1 were from a long-term captive colony maintained at the University of California, Berkeley, whereas adults from Exp. 2 were obtained from Nasco (Fort Atkinson, WI). In both experiments, three females and three males were injected with human choriogonadotropin (1,000 international units) 6 h before harvesting gametes. Eggs were manually stripped from the female and fertilized in vitro in 0.3 × modified mammalian Ringer's solution by using the sperm obtained from the dissected testes of the three males. The embryos were allowed to hatch. After 4 days, the larvae were all mixed and netted into tanks 5 at a time repeatedly, until all tanks contained 30 larvae. Larvae were reared in 4 liters of aerated 10% Holtfreter's solution (15) and fed a solution of ground Purina rabbit chow daily. Food levels were adjusted as the animals grew to maximize growth.
Dosing.
In Exp. 1, we exposed larvae to atrazine at nominal concentrations of 0.01, 0.1, 1.0, 10.0, and 25 parts per billion (ppb), whereas the second experiment used 0.1, 0.4, 0.8, 1.0, 25, and 200 ppb atrazine. Concentrations were confirmed by two independent laboratories (PTRL West, Richmond, CA, and the Iowa Hygienic Laboratory, Univ. of Iowa, Iowa City, IO). All stock solutions were made in ethanol (10 ml), mixed in 15-gallon containers, and dispensed into treatment tanks. Controls were treated with ethanol such that all tanks contained 0.004% ethanol. Water was changed and treatments were renewed once every 72 h. Each treatment was replicated 3 times with 30 animals per replicate (total of 90 animals per treatment) in both experiments. All treatments were systematically rotated around the shelf every 3 days to ensure that no one treatment or no one tank experienced position effects. Experiments were carried out at 22°C with animals under a 12-h/12-h light/dark cycle (lights on at 6 a.m.). Animals were exposed throughout the entire larval period, from hatching [Niewkwoop–Faber (NF) Stage 48 (16)] until complete tail reabsorption (NF Stage 66). In all experiments, all treatments and analyses were conducted blindly with color-coded tanks and treatments and number-coded specimens.
Gross Measurements.
At metamorphosis (complete tail reabsorption—Niewkwoop–Faber Stage 66), the date was recorded for each animal. Each animal was weighed to the nearest 0.002 g on a Mettler AT 261 Delta Range balance and its total length was measured to the nearest 0.5 mm. Animals were anesthetized in 0.2% benzocaine (Sigma), assigned a unique identification number, fixed in Bouins' fixative, and preserved in 70% ethanol until further analysis.
Gonadal Analysis.
Initially, the sex of all individuals was determined based on gross gonadal morphology (Fig. 1). Sex identification was confirmed by histology for 10 animals per tank. Further, histological analysis was conducted on all animals for which the sex was ambiguous when determined by gross morphology. All histology was conducted according to Hayes (17). In brief, tissues of interest were dissected and dehydrated in graded alcohols, followed by infiltration with histoclear and paraffin. Sections were cut at 8 μm and stained in Mallory's trichrome stain.
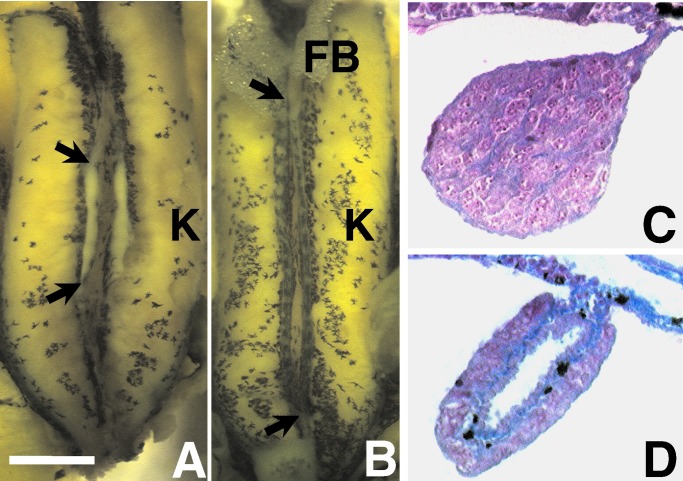
Figure 1.
Gonads of a control postmetamorphic male (A and C) and female (B and D) X. laevis. A and B show the entire dissected kidney–adrenal–gonadal complex preserved in Bouins' fixative. C and D show 8 μm of transverse cross-sections through the animals' right gonad stained with Mallory's trichrome stain. [Bar = 0.1 mm (A and B) and 10 μm (C and D)]. FB, fatbody; K, kidney. Arrows (in A and B) show the anterior and posterior ends of the animals' right gonads. The yellow color in A and B is a result of fixation in Bouins' fixative. Without fixation, the gonad is transparent. The ovary is distinguished by its greater length, lobed structure, and melanin granules. Although some specimens' ovaries lack pigment (especially atrazine-treated animals), testes never have melanin in this species. Histologically, the ovary is distinguished by the ovarian vesicle (hole in the center) along its entire length and the internal ring of connective tissue (in blue). Note the melanin granules (black) in the connective tissue in D.
Laryngeal Size.
Serial transverse histological sectioning was conducted on the larynges of 10 males and 10 females from each replicate from all treatments in both experiments. Histology was conducted as described above. To estimate the size of the larynx, the M. dilator laryngis was measured. We used the largest cross-sectional area (transverse section) as a measure of muscle size. Initially, 10 sections were taken from 100 animals (distributed over all treatments from Exp. 1) until a region approximately one-third through the larynx was repeatedly determined to be the largest section. For the final analysis this region was identified by shape. Thus, similar sections were measured for each individual. Images of this section from each animal were recorded with a Sony DKC-5000 and analyzed with metamorph software (version 2.75, Universal Imaging, Media, PA).
Adult Treatments.
Newly metamorphosed animals were too small to obtain enough plasma to measure hormone levels. Thus, studies of effects of atrazine on hormone levels focused on adults. For adult studies, males and females were obtained from a long-term captive colony at University of California, Berkeley. Adults were maintained under the same light and temperature cycles as described for larvae. Animals were acclimated in 10% Holtfretter's solution for 5 days and then exposed to 25 ppb atrazine. Water was not aerated, animals were fed Purina trout chow daily, and water was changed and treatment renewed every 72 h. Animals were treated for 46 days. At the end of the exposure, animals were killed by decapitation, and the blood was collected. Plasma was collected and stored frozen until analysis.
RIA.
For testosterone analysis, plasma was extracted with diethyl ether and dried under nitrogen. All samples were reconstituted in PBS with gelatin (PBS-g). Hormone assays were conducted as described in Hayes and Licht (18). Testosterone antisera were obtained from Endocrine Sciences (Calabasas, CA) and were validated for several species including Xenopus laevis. Plasma from controls and treated animals was assayed in the same assay at 3 doses and the assay was repeated 3 times. Intraassay variation was 1.0%, and interassay variation was 1.3%.
Statistical Analysis.
Statistical analysis was conducted with the aid of systat software (SPSS, Chicago). Sex ratios were analyzed by using the G test with Wilkin's g- adjustment as described in Hayes and Menendez (19). Similarly, mortality was analyzed by using the G test. Time to metamorphosis and size (length and weight) at metamorphosis were analyzed by using ANOVA with treatment, tank, and sex (sex nested within tank and tank nested within treatment) as independent variables. In addition, we conducted correlational analyses to determine whether laryngeal size correlated with time to metamorphosis, size, or atrazine dose. Also, we scored all animals as to whether they were greater or less than the mean laryngeal size for controls and then conducted a G test to determine whether the number of affected animals in the treatment group changed with atrazine treatment. Finally, we used Kendall's ranked coefficient to determine whether the percentage of below-average animals varied with the dose of atrazine.
Results
Mortality, Development, and Growth.
At the doses tested, atrazine exposure had no effects (P > 0.05) on mortality, time to metamorphosis, length, or weight at metamorphosis (not shown).
Effects on Primary and Secondary Sex Differentiation.
Males and females were sexually differentiated at metamorphosis based on gonadal morphology and histology (Fig. 1). At all doses tested (except 0.01 ppb), atrazine produced gonadal abnormalities. Up to 20% of the animals (16–20%) had multiple gonads (up to 6 in a single animal) or were hermaphrodites (with multiple testes and ovaries; Fig. 2). These abnormalities were never observed in control animals in the current experiments or in over 10,000 observations of control animals in our laboratory over the last 6 years.
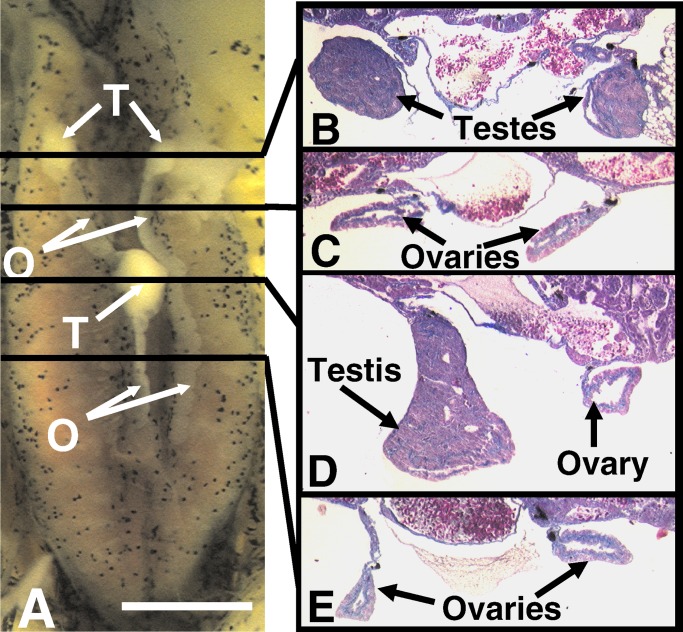
Figure 2.
An atrazine-treated hermaphrodite. The specimen shown was treated with 1 ppb atrazine. A shows the entire dissected kidney–adrenal–gonadal complex. B–E show 8 μm of transverse cross-sections (stained with Mallory's trichrome stain) through the areas indicated by the lines in A. [Bar = 0.1 mm (A) and 25 μm (B–E)]. FB, fatbody; K, kidney; O, ovary(ies); T, testis(es). Note the absence of pigment in the ovaries, which was typical of hermaphrodites.
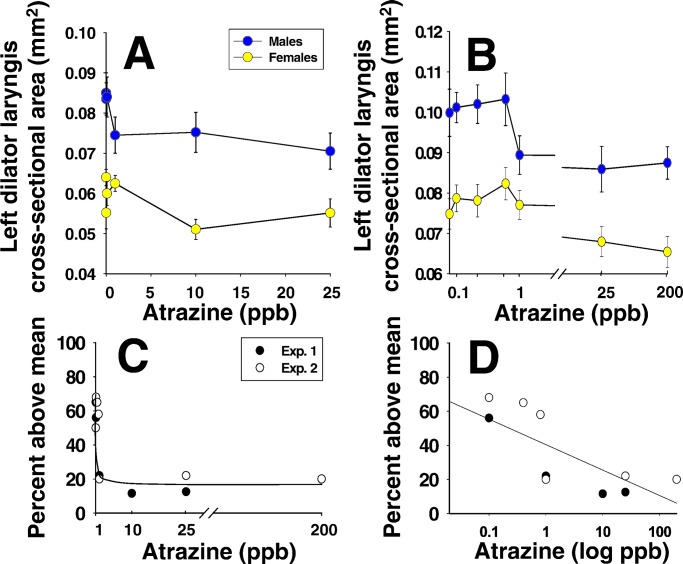
Control males had larger larynges than females at metamorphosis, but males exposed to atrazine (≥1 ppb) had reduced larynges (both studies; Fig. 3 A and B). When we examined the proportion of “below-average” animals against dose, we found a threshold effect at 1 ppb (both studies; Fig. 3C), but Kendall's rank coefficient suggested a dose effect with increasing proportions of affected males associated with increasing atrazine doses (P < 0.01; Fig. 3D).
Figure 3.
Results of measurements of the left laryngeal muscle (M. dilator laryngis) in control males and females compared with atrazine-treated animals. In Exp. 1 (A), atrazine (≥1 ppb) reduced laryngeal size in males but did not affect females. Doses of 0.01 and 0.1 ppb did not have a significant effect. In Exp. 2 (B), 0.1–0.8 ppb atrazine did not have a statistically significant effect on laryngeal size but again, exposure to ≥1 ppb atrazine significantly reduced laryngeal size in males (P < 0.05). Laryngeal size was greater in animals from Exp. 2 compared with Exp. 1, suggesting a population difference in the absolute size of the larynges, but the relative sizes (male to female and atrazine-treated compared with controls) were similar within each experiment. C and D show two interpretations of the data by using analysis of the proportion of above-average males for both experiments. Atrazine exposure (≥1 ppb) significantly decreased the proportion of males that were at or above the mean for control males (G test; P < 0.05) and suggested a threshold effect at 1.0 ppb in which 80% of the exposed males were below average (C). Kendall's rank coefficient analysis (P < 0.01), however, suggested a relationship between dose and the proportion of affected males with a decrease in the proportion of normal males with increased dose (D). Note that control males were normally distributed with exactly 50% of the individuals above the mean in both experiments.
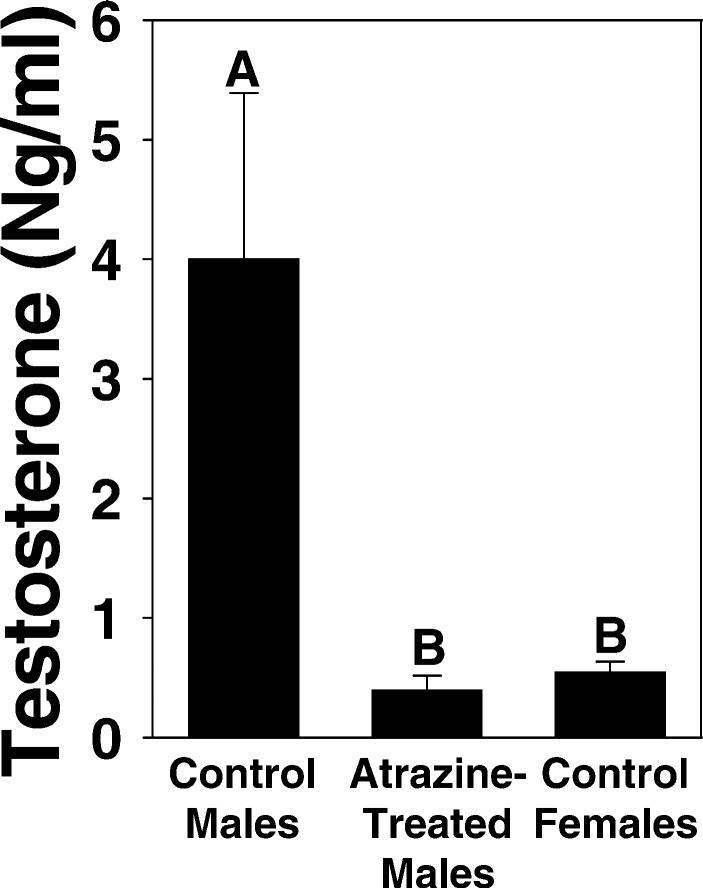
We hypothesized that the effects of atrazine were caused by a disruption of steroidogenesis (20–27). Further, we showed that sexually mature males suffered a 10-fold decrease in plasma testosterone (Fig. 4).
Figure 4.
Effect of 46-day exposure to atrazine on plasma testosterone levels in sexually mature male X. laevis. Sexually mature males were housed individually. Experimental animals were treated every 3 days with 25 ppb atrazine, and controls were treated identically except without atrazine exposure. Control females are shown for comparative purposes. Letters above bars show statistical groupings (ANOVA, P < 0.05).
Discussion
Although data from two experiments are reported here, these studies have been repeated four times, including an unpublished report and a study submitted to the U.S. Environmental Protection Agency (28). In total, atrazine exposure at these levels has been replicated 51 times by our laboratory with similar results. We chose X. laevis for these studies, because it is a well studied laboratory model for which the effects of sex steroids are well known. Exposure to exogenous estrogen in this species results in 100% females (29, 30), whereas androgens increase laryngeal growth but do not affect gonadal differentiation (30, 31). Thus, endpoints for detecting sex steroid-like or antagonistic effects are well defined for this species. The current findings suggest that atrazine inhibits testosterone and induces estrogen secretion.
Previous studies have suggested that atrazine is an endocrine disruptor, but these effects have been observed in a single strain of rat or were produced only at high doses (32–38). In fact, no published studies have addressed effects of atrazine at concentrations considered safe in drinking water or safe for limited human exposure—3 and 200 parts ppb, respectively (39). Also, until now, the potential endocrine-disrupting effects of atrazine have not been examined in amphibians, although teratogenesis, mortality, and growth effects have been examined at high doses (14, 40–45). In the cited amphibian studies, deformities, acute toxicity, or physiological impairments were not detected below atrazine doses of 47.6 ppm.
Disruption of steroidogenesis by atrazine has been reported in mammals (20–26) and reptiles (27), however. Several of these studies reported the induction of aromatase and an increase in estrogen. Here, we suggest that the same mechanism may explain the effects observed in X. laevis. An induction of aromatase may result in the decrease in androgens (as androgens are the substrate for aromatase). The loss of masculine features, such as the decreased laryngeal size, may be a result of the decreased androgens, whereas the induction of ovaries may be a result of increased estrogen synthesis and secretion. The possible common mechanism underlying the abnormal sexual development in the current study and reproductive abnormalities in reptiles and mammals has significant implications for environmental and public health. The effects observed in mammals were dismissed as a concern for public health because the exposure levels were very high (20–26, 32–38). The effective doses in the current study, however, demonstrate the sensitivity of amphibians relative to other taxa, validate the use of amphibians as sensitive environmental monitors/sentinels, and raise real concern for amphibians in the wild. The effects on the gonads in the current study were produced at 0.1 ppb, which was more than 600 times lower than the dose required to induce aromatase in human adrenocortical carcinoma (25) and placental choriocarcinoma studies (25–26) and 30,000,000 times lower than the dose required to produce reproductive effects in rats (24).
Furthermore, the current data demonstrate the importance of considering endocrine-regulated endpoints in assessing the potential impact of pesticides on amphibians. Reported teratogenesis, growth inhibition, and mortality in amphibians in response to atrazine were not considered environmental concerns because of the high doses required to produce these effects (40). Effects in the current study, however, occurred at levels 10,000 times lower than the dose required to produce effects in amphibians in these previous studies (40–45). Allran and Karasov (14) reached the conclusion that atrazine was probably not a significant factor in amphibian declines based on their studies of toxicity, deformities, and effects on feeding and ventilation in leopard frogs that did not produce noticeable effects below 3 ppm. The current data show that negative effects on sex differentiation occur at doses 30,000 times lower than effective doses reported by Allran and Karasov. The Allran and Karasov study, however, examined a different species and different endpoints.
The current data raise new concerns for amphibians with regards to atrazine. Effective doses (0.1 ppb for the production of hermaphrodites and 1 ppb for reduction in laryngeal size) are ecologically relevant. The recommended application level of atrazine ranges from 2,500,000–29,300,000 ppb (46), the allowable contaminant level for atrazine in drinking water is 3 ppb (39), and short-term exposures of 200 ppb are not considered a health risk. Atrazine can be as high as 21 ppb in ground water, 42 ppb in surface waters, 102 ppb in river basins in agricultural areas, up to 224 ppb in Midwestern streams, and up to 2,300 ppb in tailwater pits in Midwestern agricultural areas (47, 48). Atrazine can be found in excess of 1 ppb in precipitation in localities where it is not used and up to 40 ppb in rainfall in Midwestern agricultural areas (49–51). Further, Davidson et al. (52) recently reported that at least one species (Rana aurora) may be affected by aerial transport of agrichemicals. They showed that declines and extirpations of R. aurora populations were strongly correlated with areas that were downwind of agricultural activity. Furthermore, Cory et al. (53) showed that agrichemicals can be transported aerially and accumulated in amphibians' tissues. Thus, the likelihood that wild amphibians are exposed to 0.1 ppb or even 1 ppb atrazine is extremely high.
Furthermore, atrazine is typically applied when the soil is tilled, such that levels are highest during spring rainfall (13). This pattern of use puts amphibians at great risk, because the highest atrazine levels coincide with the breeding season for amphibians. Throughout areas where atrazine is used, atrazine levels peak while larval amphibians are at critical developmental stages. Also, depending on the species, amphibians breed in every possible freshwater microhabitat—from temporary pools, irrigation ditches, and flooded fields, to streams, rivers, lakes, and other permanent sources of water. The current data raise the question of the threat of atrazine, in particular, and of pesticides, in general, to amphibians in the wild. Low-dose endocrine-disrupting effects, which have not been addressed extensively in amphibians, are of special concern in this regard. If such effects do occur in the wild in other species, exposed animals could suffer impaired reproductive function. The described effects are all internal and may go unnoticed by researchers—unlike mortality and external malformations. Thus, exposed populations could decline and even go extinct without any recognition of the developmental effects on individuals. Already, it has been suggested that pesticides may play a role in amphibian declines (3, 52, 54, 55). Also, Reeder et al. (56) found that atrazine exposure may be associated with intersexual cricket frogs in the wild in the Illinois. Because the P value in the Reeder et al. study was 0.07 and because no laboratory data were available, they concluded that “[w]hether atrazine accounts for findings of intersexuality is less clear” (ref. 56, p. 265). We believe that the current data strongly suggest a connection between atrazine exposure and intersexuality. Combined with the decreases in dissolved oxygen, pH, and available food sources (phytoplankton, periphyton, and macrophytes) caused by atrazine (45), this common contaminant could be a contributing factor in amphibian declines. Ongoing investigations of the effects of atrazine on other species and amphibians in the wild will assess the realized role of this widespread compound in amphibian declines.
Acknowledgments
We thank Nadir Yeyah for animal breeding and Diana Reyes for assistance with data collection. The following people assisted with histological analysis and data collection: Adrian Brunner-Brown, Karen Chan, Sarah Chui, Anu Devi, Kelly Haston, Isabel Hsu, Gwynne Johnston, Roger Liu, Emily Marquez, and Mable Tsui. We thank Anhthu Hoang for comments on experimental design, analysis, and manuscript preparation. We thank Katherine Kim (Sokoke) for her support. All work was conducted in compliance with animal use protocol no. R209-0402BCR to Hayes. This work was funded by a grant from the National Science Foundation (IBN-9513362), and by the Biology Faculty Award, University of California, Berkeley (to T.B.H.). N.N. was a Presidential Fellow (University of California, Berkeley).
Abbreviation
- ppb
parts per billion
References
- 1.Sonnenschein C, Soto A M. J Steroid Biochem Mol Biol. 1998;65:143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 2.Cooper R L, Goldman J M, Stoker T E. Toxicol Ind Health. 1999;15:26–36. doi: 10.1177/074823379901500104. [DOI] [PubMed] [Google Scholar]
- 3.Wake D B. Science. 1991;253:860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- 4.Houlahan J E, Findlay C S, Schmidt B R, Meyer A H, Kuzmin S L. Nature (London) 2000;404:752–755. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- 5.Kiesecker J M, Blaustein A R, Belden L K. Nature (London) 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- 6.Ankley G, Mihaich E, Stahl R, Tillitt D, Colborn T, McMaster S, Miller R, Bantle J, Campbell P, et al. Environ Toxicol Chem. 1998;17:68–87. [Google Scholar]
- 7.Tyler C R, Jobling S, Sumpter J P. Critic Rev Toxicol. 1998;28:319–361. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- 8.Younes M. Chemosphere. 1999;39:1253–1257. doi: 10.1016/s0045-6535(99)00193-9. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Nakadaira H, Nakamura K, Endoh K. Biomed Res (Tokyo) 2000;21:361–367. [Google Scholar]
- 10.Oberdorster E, Cheek A O. Environ Toxicol Chem. 2001;20:23–36. [PubMed] [Google Scholar]
- 11.Ashby J. Toxicol Pathol. 2000;28:432–437. doi: 10.1177/019262330002800312. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Dept. Agric. Pesticides Industry Sales and Usage: 1992 and 1993 Market Estimates. Environ. Protect. Agency; 1994. , U.S. Dept. Agric. Publ. No. 733-K-94-001. [Google Scholar]
- 13.Solomon K, Baker D B, Richards R P, Dixon K R, Klaine S J, La Point Thomas, W, Kendall R J, Weisskopf C P, Giddings J M, et al. Environ Toxicol Chem. 1996;15:31–76. [Google Scholar]
- 14.Allran J W, Karasov W H. Environ Toxicol Chem. 2001;20:769–775. doi: 10.1897/1551-5028(2001)020<0769:eoaoel>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Holtfreter J. Arch F Ent Mech. 1931;124:404–465. doi: 10.1007/BF00652482. [DOI] [PubMed] [Google Scholar]
- 16.Niewkwoop P D, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North Holland Publishing; 1994. [Google Scholar]
- 17.Hayes T B. J Morphol. 1995;226:297–307. doi: 10.1002/jmor.1052260306. [DOI] [PubMed] [Google Scholar]
- 18.Hayes T B, Licht P. J Exp Zool. 1992;264:130–135. [Google Scholar]
- 19.Hayes T B, Menendez K P. Gen Comp Endocrinol. 1999;115:188–199. doi: 10.1006/gcen.1999.7321. [DOI] [PubMed] [Google Scholar]
- 20.Babić-Gojmerac T, Kniewald Z, Kniewald J. J Steroid Biochem. 1989;33:141–146. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- 21.Kniewald J, Osredec̆ki V, Gojmerac T, Zechner V, Kniewald Z. J Appl Toxicol. 1995;15:215–218. doi: 10.1002/jat.2550150312. [DOI] [PubMed] [Google Scholar]
- 22.Danzo B J. Environ Health Perspect. 1997;105:306–310. doi: 10.1289/ehp.97105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldridge J C, Fleanor-Heyser D G, Extrom P C, Wetzel L T, Breckenridge C B, Gillis J H, Luemperti L G, Stevens J T. J Toxicol Environ Health. 1994;43:155–168. doi: 10.1080/15287399409531912. [DOI] [PubMed] [Google Scholar]
- 24.Wetzel L T, Luempert L G, 3rd, Breckenridge C B, Tisdel M O, Stevens J T, Thakur A K, Extrom P J, Eldridge J C. J Toxicol Environ Health. 1994;43:169–172. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson J T, Seinen W, Giesy J P, van den Berg M. Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- 26.Sanderson J T, Letcher R J, Heneweer M, Giesy J P, van den Berg M. Environ Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craine D A, Guillette L J, Jr, Rooney A A, Pickford D B. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parshley T. Report of an Alleged Adverse Effect from Atrazine: Atrazine Technical. Environ. Protect. Agency; 2000. , Environ. Protect. Agency Reg. No.100-529. [Google Scholar]
- 29.Gaillien L. Bull Biol Fr Belg. 1962;90:163–183. [Google Scholar]
- 30.Hayes T B. J Exp Zool. 1998;281:373–399. [PubMed] [Google Scholar]
- 31.Sassoon D G, Gray G, Kelley D B. J Neurosci. 1987;7:3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldridge J C, Tennant M K, Wetzel L T, Breckenridge C B, Stevens J T. Environ Health Perspect. 1994;102,Suppl.:29–36. doi: 10.1289/ehp.94102s1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper R L, Stoker T E, Goldman J M, Parrish M B, Tyrey L. Reprod Toxicol. 1996;10:257–264. doi: 10.1016/0890-6238(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 34.Eldridge J C, Wetzel L T, Stevens J T, Simpkins J W. Steroids. 1999;64:672–678. doi: 10.1016/s0039-128x(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 35.Eldridge J C, Wetzel L T, Tyrey L. Reprod Toxicol. 1999;13:491–499. doi: 10.1016/s0890-6238(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 36.Cooper R L, Stoker T E, Tyrey L, Goldman J M, McElroy W K. Toxicol Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- 37.Cummings A M, Rhodes B E, Cooper R L. Toxicol Sci. 2000;58:135–143. doi: 10.1093/toxsci/58.1.135. [DOI] [PubMed] [Google Scholar]
- 38.Kniewald J, Jakominic M, Tomljenovic A, Simic B, Romal P, Vranesic D, Kniewald Z. J Appl Toxicol. 2000;20:61–68. [PubMed] [Google Scholar]
- 39.Hayes E. EPA J. 1993;19:48–49. [Google Scholar]
- 40.Morgan M K, Scheuerman P R, Bishop C S, Pyles R A. J Toxicol Environ Health. 1996;48:151–168. doi: 10.1080/009841096161401. [DOI] [PubMed] [Google Scholar]
- 41.Clements C, Ralph S, Petras M. Environ Mol Mutagen. 1997;29:277–288. doi: 10.1002/(sici)1098-2280(1997)29:3<277::aid-em8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Howe G E, Gillis R, Mowbray R C. Environ Toxicol Chem. 1998;17:519–525. [Google Scholar]
- 43.Britson C A, Threlkeld S T. Bull Environ Contam Toxicol. 1998;61:154–161. doi: 10.1007/s001289900742. [DOI] [PubMed] [Google Scholar]
- 44.Britson C A, Threlkeld S T. J Iowa Acad Sci. 2000;107:61–66. [Google Scholar]
- 45.Diana S G, Resetarits W J, Jr, Schaeffer D J, Beckmen K B, Beasley V R. Environ Toxicol Chem. 2000;19:2961–2967. [Google Scholar]
- 46.Ciba-Geigy. Aatrex Product Booklet. Mississauga, ON, Canada: Ciba-Geigy Canada; 2001. [Google Scholar]
- 47.Kolpin D W, Sneck-Fahrer D, Hallberg G R, Libra R D. J Environ Qual. 1997;26:1007–1017. [Google Scholar]
- 48.Battaglin W A, Furlong E T, Burkhardt M R, Peter C J. Sci Total Environ. 2000;248:123–133. doi: 10.1016/s0048-9697(99)00536-7. [DOI] [PubMed] [Google Scholar]
- 49.Nations B K, Hallberg G R. J Environ Qual. 1992;21:486–492. [Google Scholar]
- 50.van Dijk H F G, Guichert R. Water Air Soil Pollut. 1999;115:21–70. [Google Scholar]
- 51.Thurman E M, Cromwell A E. Environ Sci Technol. 2000;34:3079–3085. [Google Scholar]
- 52.Davidson C, Shaffer H B, Jennings M R. Ecol Appl. 2001;11:464–479. [Google Scholar]
- 53.Cory L, Fjerd P, Serat W. Pestic Monit J. 1970;3:204–211. [PubMed] [Google Scholar]
- 54.Hayes T B. In: Herpetologia Bonnensis. Böhme W, Bischoff W, Ziegler T, editors. Bonn: SEH; 1997. pp. 145–150. [Google Scholar]
- 55.Hayes T B. In: Ecotoxicology in Reptiles and Amphibians. Linder G, Sparling D, Bishop C, editors. Pensacola, FL: Soc. Environ. Toxicol. Chem.; 1999. pp. 573–594. [Google Scholar]
- 56.Reeder A L, Foley G L, Nichols D K, Hansen L G, Wikoff B, Faeh S, Eisold J, Wheeler M B, Warner R, Murphy J E, Beasley V R. Environ Health Perspect. 1998;106:261–266. doi: 10.1289/ehp.98106261. [DOI] [PMC free article] [PubMed] [Google Scholar]