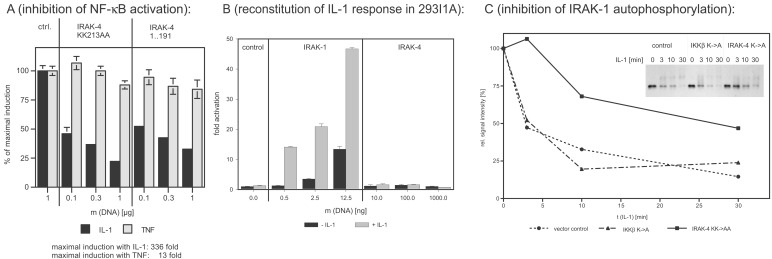
Figure 4.
Dominant-negative effects of IRAK-4 mutants and reconstitution experiments in IRAK-1-deficient 293 cells. (A) Inhibition of IL-1-induced NF-κB activation. The 293RI cells were transfected with an NF-κB-dependent ELAM-luc reporter gene construct, pRSVβgal for normalization, and the indicated amounts of kinase-inactive IRAK-4 (IRAK-4 KK213AA) and truncated IRAK-4 (amino acids 1–191). Twenty-four hours after transfection, cells were stimulated with 50 ng/ml of IL-1β or 100 ng/ml of tumor necrosis factor α for 6 h, and luciferase and β-galactosidase activity were determined. The y axis represents the percentage of NF-κB activation relative to cells transfected with empty vector. (B) Reconstitution of IL-1 response in 293I1A. IRAK-1-deficient 293 cells [293I1A(16)] were transfected and stimulated as described for Fig. 4A. The y axis represents the fold of NF-κB activation relative to cells transfected with empty vector. (C) Inhibition of IL-1-induced IRAK-1 activation. The 293RI cells were transfected with control vector, kinase-inactive IKKβ (negative control), or kinase-inactive IRAK-4 for 24 h. After stimulation with 100 ng/ml of IL-1β for the indicated amount of time, the cells were lysed, and IRAK-1 was immunoprecipitated with the mAb 2A9. The activation level of IRAK-1 was assessed by determining the shift in apparent molecular weight and subsequent degradation of IRAK-1 by Western blotting (Inset). The signal intensity was quantified by densitometry and plotted as a function of incubation time.