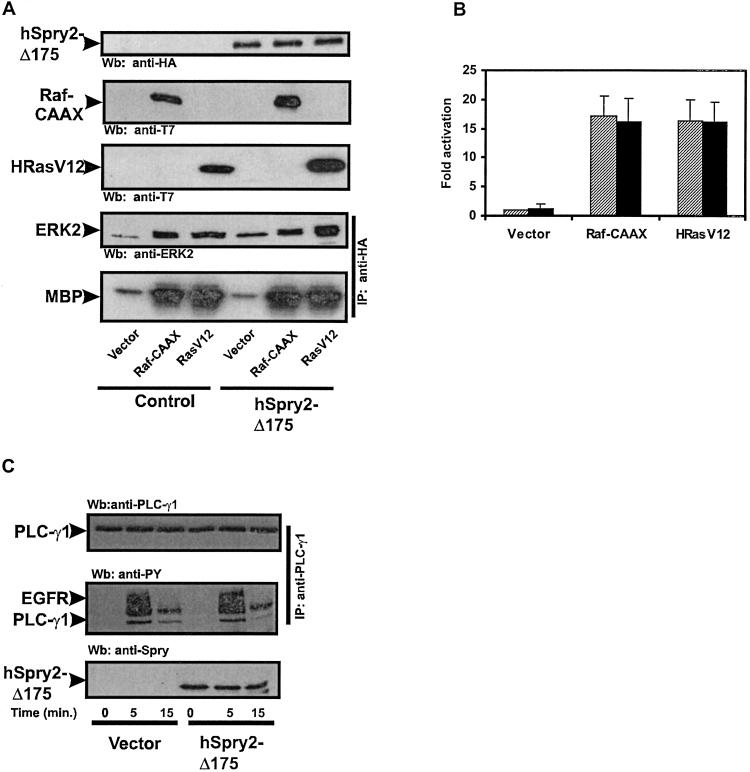
Figure 3.
The cysteine-rich domain of hSpry2 acts upstream of Ras. (A) HeLa cells cotransfected with HA-ERK2 in the absence or presence of HA-hSpry-Δ175, and vector, T7-Raf-CAAX, or T7-HRasV12. Transfected cells were serum-deprived for 16 h, and MAP kinase activation was measured by an immunocomplex kinase assay with MBP as a substrate. Expression levels of ectopically expressed proteins were determined by the Wb of cell lysates with the indicated Abs. (B) Quantitation of the level of MAP kinase activity induced by the transfection of vector, T7-Raf-CAAX, or T7-HRasV12 in the absence (shaded bars) or presence of HA-hSpry2-Δ175 (solid bars). The amount of phosphorylated MBP was determined with a PhosphorImager and is presented in arbitrary units as the fold activation over serum-deprived vector-expressing cells. Shown are the averages of five independent experiments ± SDs. (C) Serum-starved HeLa cells coexpressing either vector or HA-hSpry2-Δ175 and human PLC-γ1 were stimulated with 10 ng/ml of EGF for the indicated times. PLC-γ1 was immunoprecipitated (IP) with anti- PLC-γ1 Ab and detected by Wb with antiphosphotyrosine Ab (PY). The slower migrating tyrosine-phosphorylated band corresponds to the activated EGFR that was coprecipitated with PLC-γ1. Expression levels of HA-hSpry2-Δ175 or PLC-γ1 were determined by using anti-HA and anti-PLC-γ1 Abs, respectively. The results shown are from a single experiment that is representative of four independent experiments.