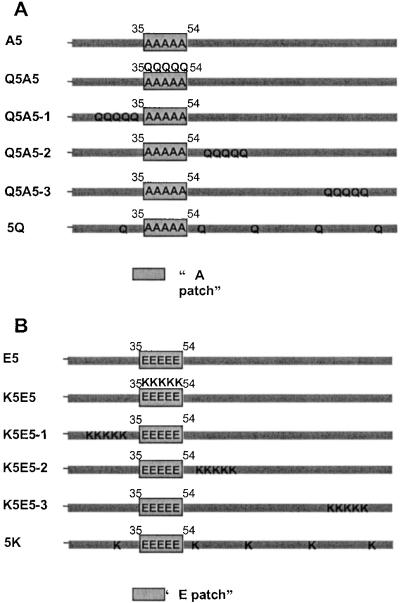
Figure 2.
(A) Patch and dispersed locations of K to Q mutations. In A5, the five phosphorylation sites were changed to alanine, producing a constitutively unphosphorylated H1. Starting with A5, in Q5A5, five lysines in the region of the phosphorylation sites were changed to glutamines, reducing the positive charge without introducing any negatively charged molecules that resemble phosphates. Q5A5-1, -2, and -3 are like Q5A5 except that five lysines on the N-terminal side of the phosphorylation region, at the immediate C-terminal side of the phosphorylation region and in the C-terminal region of H1, were changed to glutamines. In 5Q, five lysines (≈30 aa apart) were changed to glutamines. The net charges of the Q5A5, Q5A5-1, -2, and –3, and 5Q versions of H1 were the same, similar to that of E5. (B) Patch and dispersed locations of neutral to K mutations. In E5, the five phosphorylation sites were changed to glutamic acids, mimicking a constitutively phosphorylated H1. Starting with E5, in K5E5 five neutral residues in the region of the phosphorylation sites were changed to lysines, introducing positive charges. K5E5-1, -2, and -3 are like K5E5 except that five neutral residues at the N-terminal side of the phosphorylation region, at the immediate C-terminal side of the phosphorylation region, and in the C-terminal region of H1 were changed to lysines. In 5K, five neutral residues (≈30 aa apart) were changed to lysines. The net charges of the K5E5, K5E5-1, -2, and –3, and 5K versions of H1 were the same, similar to that of A5.