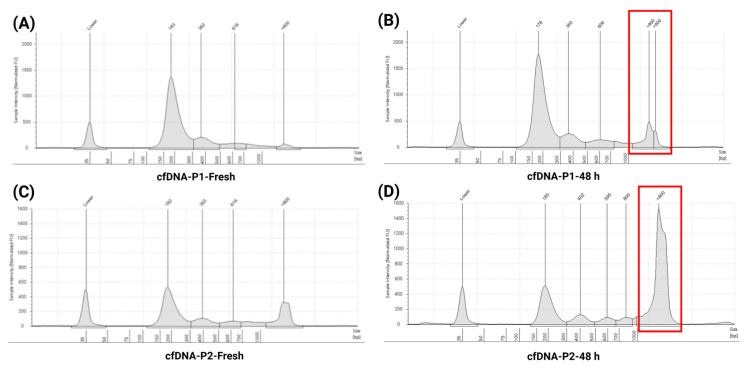
Figure 9.
Assessment of cfDNA stability in blood samples stored at RT for up to 48 h compared to freshly processed samples. (A,C) TapeStation electropherograms of cfDNA extracted from freshly processed clinical blood samples, showing distinct cfDNA fragment peaks with minimal gDNA contamination. (B,D) Electropherograms of cfDNA extracted from matched clinical samples stored at RT for up to 48 h, demonstrating characteristic cfDNA fragment peaks around ~180 bp (mononucleosomal), ~370–380 bp (dinucleosomal), and ~600 bp (trinucleosomal), accompanied by marked gDNA contamination (>800 bp), as highlighted in the red boxes. These findings underscore the adverse impact of prolonged RT storage on cfDNA integrity and purity, and highlight the importance of timely plasma processing to minimize pre-analytical variability.