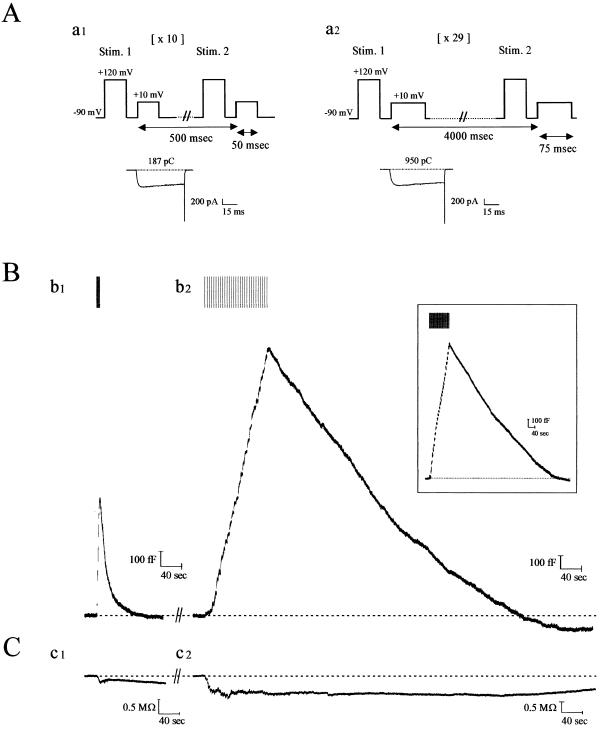
Figure 1.
Kinetics of endocytosis are altered with degree of stimulation of calf chromaffin cells. (A) Patch-clamped calf chromaffin cells were stimulated with depolarizing pulse trains of 10 × 50 msec (with 0.5 sec between each stimulus; a1) or 29 × 75 msec (with 4 sec between each stimulus; a2). Before each depolarization, a prepulse was administered to recruit facilitation L-type Ca2+ channels in this preparation (9). Typical Ca2+ currents recorded from the first depolarizations in a1 and a2 are shown below the protocols (numbers indicate cumulative Ca2+ entry for each protocol; note this is 5.5-fold larger for the sustained versus the transient paradigm). (B) Continuous Cm recordings after formation of whole-cell configuration from a single calf chromaffin cell, sequentially stimulated with the two protocols illustrated in A. (b1) With transient stimulation, exocytosis (rising phase of Cm trace) was immediately followed by RE (falling phase of Cm trace). (b2) After a 12-min recovery period, during which baseline Cm was attained and remained stable, a second round of secretion was elicited with the sustained protocol. Under these conditions, endocytosis was slow, and Cm returned to baseline in ≈9 min (see Table 1 for average values). (Inset) SE was evoked during the first round of stimulation, 3 min after establishing whole-cell configuration. Results from a typical experiment are shown [mean values (n = 12) were peak current (676 ± 44 pA), current integral (767 ± 36 pC), total Cm increase (1,750 ± 125 fF), rate of endocytosis (134 ± 20 fF/min) and endocytosis duration (10 ± 0.6 min), respectively]. (C) Conductance traces accompanying the records in B show that this parameter does not change in parallel and thus does not significantly contaminate the Cm records. In total, >90% of the cells tested expressed both forms of endocytosis.