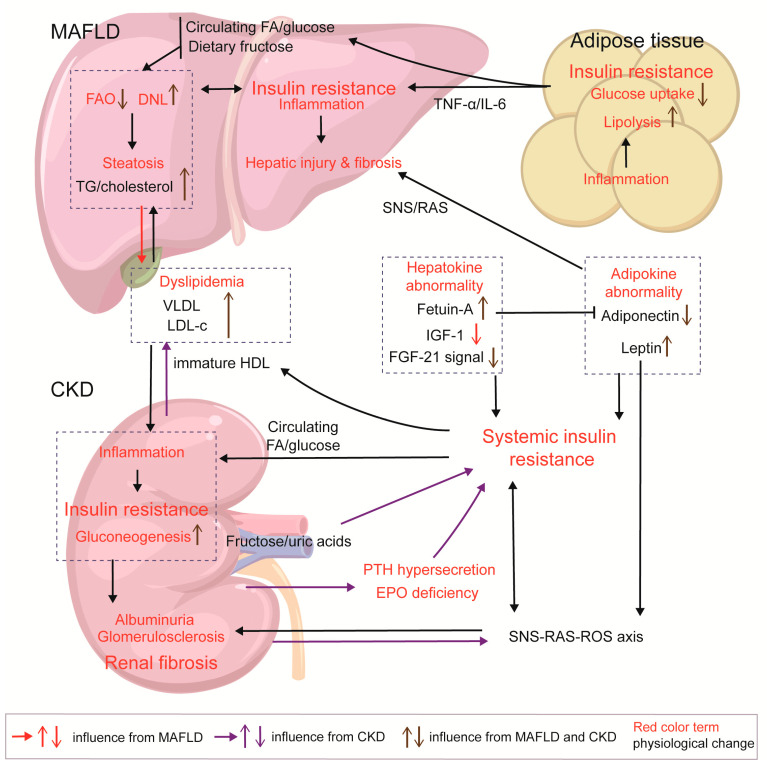
Figure 4.
Insulin resistance in MAFLD and CKD and the interaction between the two diseases. In NAFLD, lipid overload triggers inflammatory responses and insulin resistance in adipocytes, acting as a pump for the export of FFA/glucose and inflammatory factors. Hepatic insulin resistance can be driven by high-fructose diets, dysregulation of FAO/DNL, and the formation of lipotoxic products, as well as by increased energy input from peripheral tissues. In CKD, HDL immaturity, increase in fructose/uric acid axis, decreased EPO production, and PTH hypersecretion contribute to systemic insulin resistance. CKD and diabetes can both cause HDL immaturity, leading to reduced HDL-c and increased LDL-c levels; meanwhile, MAFLD enhances VLDL output, exacerbating this pattern of dyslipidemia. Increased deposition of lipoproteins (VLDL and LDL-c), FAs, and glucose in the kidneys induces lipotoxicity and inflammatory response, which stimulate gluconeogenesis activation and insulin resistance in the kidneys; insulin resistance in the kidneys exacerbates glomerulosclerosis and renal fibrosis. MAFLD and CKD both exhibit abnormalities in adipokines and hepatokines, which induce systemic insulin resistance; abnormal adipokines cause damage to glomerulus and liver cells. Within the SNS/RAS/ROS axis, insulin, and adipokine, there is a feedback loop amplifying the interactions among them, and then leptin/SNS/RAS axis activates fibrotic signals in the liver and kidneys.