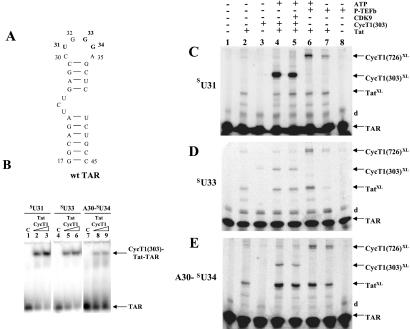
Figure 1.
Site-specific photocross-linking in CycT1–Tat-TAR complexes containing 4-thiouridine. (A) Secondary structure of TAR RNA used in this study. TAR RNA spans the minimal sequences required for Tat responsiveness in vivo (32) and for in vitro binding of Tat-derived peptides (33). Wild-type (wt) TAR contains two non-wild-type base pairs to increase transcription by T7 RNA polymerase. Sites of 4-thiouridine incorporation, U31, G33, and G34, are bolded in the TAR RNA. Numbering of nucleotides in the RNA corresponds to their positions in wild-type TAR RNA. (B) RNA gel-shift analysis of complexes containing recombinant Tat, CycT1(1–303), and sU31, sU33, and A30–sU34 TAR RNA sequences. Control (C) lanes are without proteins. Lanes 2 and 3, 5 and 6, and 8 and 9 contain increasing concentrations of protein complexes (0.2 μg of Tat and 0.6–1.2 μg of CycT1). Arrows indicate the position of RNA and RNA–protein complexes. (C–E) Analysis of RNA–protein photocross-link products. TAR RNA containing sU at specific positions in the loop was 5′ end-labeled and used to form complexes with Tat and CycT1 or P-TEFb, UV-irradiated (360 nm), and resolved on 15% SDS polyacrylamide gels. The sU RNA used is indicated on the left, and identities of cross-linked products are on the right. The letter “d” refers to dimers of TAR RNA. Lane 1 (control) contains RNA without irradiation. RNA–protein cross-link products with Tat, CycT1(1–303), and full-length CycT1 from P-TEFb are indicated as TatXL, CycT1 (303)XL, and CycT1 (726)XL, respectively.