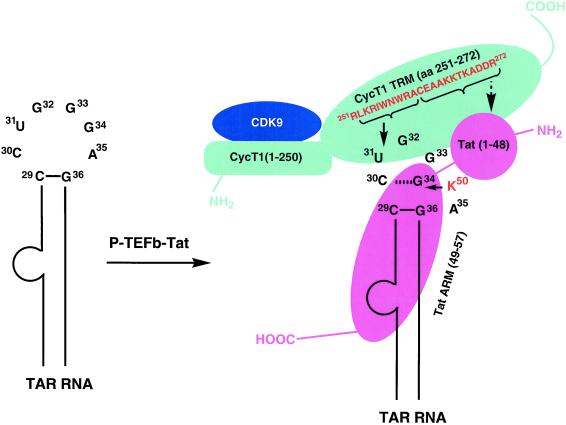
Figure 6.
Schematic representation of P-TEFb–Tat-TAR ternary complex. P-TEFb is a two-subunit kinase complex containing a CDK9 and a CycT1. CDK9 subunit of P-TEFb binds to the cyclin box sequence (amino acids 1–250) of CycT1. A Tat:TAR recognition motif (TRM) in the CycT1 sequence spans amino acid residues 251–272, which is necessary to form a complex with Tat and TAR RNA. Amino acid sequence 252–260 from TRM was cross-linked to the U31 side of the TAR loop, suggesting that the residues 261–272 are involved in interaction with Tat core domain (amino acids 1–48), shown as a round ball indicating a compact structure. RNA is recognized by the Arg-rich RNA recognition motif (ARM) of Tat, and Lys-50 interacts with the G34 side of the TAR loop. CycT1 and Tat binding to TAR RNA is highly cooperative and a capacity of 85%, Hill coefficient of 2.7, and dissociation constant (KD) of 2.45 nM were observed (18), indicating that there are three binding sites on TAR RNA. It is conceivable that the CycT1–Tat heterodimer directly binds to TAR RNA in the U-rich RNA bulge region, and this binding facilitates the interactions of CycT1–Tat at the other two sites in the RNA loop: CycT1 interacts with the U31 side, and Tat binds to the G34 side. Amino acids cross-linked with TAR RNA are shown by solid arrows and the suggested Tat-interacting region of TRM is shown by a broken arrow. CycT1 is shown in cyan, CDK9 in blue, and Tat in pink. N and C termini of proteins are labeled as NH2 and COOH, respectively.