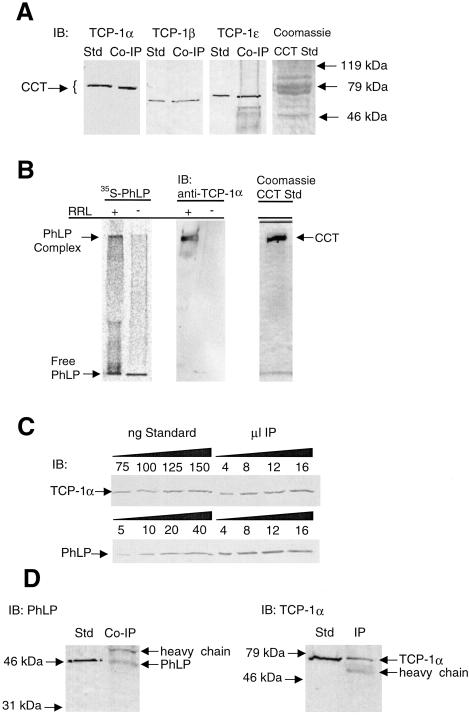
Figure 3.
Confirmation of the PhLP⋅CCT interaction and determination of the stoichiometry of binding. (A) Immunoprecipitates identical to those from Fig. 1A were immunoblotted with antibodies to TCP-1α, β, and ɛ. Purified CCT was used as a standard in each blot. (B) 35S-PhLP (50 nM) was incubated with or without 10% rabbit reticulocyte lysate (RRL) containing CCT and incubated for 30 min at 30°C. Samples were run on a 4% native gel. (Left) A visualization of the 35S-PhLP from the PhosphorImager. (Center) An immunoblot (IB) for TCP-1α. (Right) A Coomassie blue stain of purified CCT run under the same conditions. The PhLP⋅CCT complex entered the gel and migrated at an Rf value of 0.11, whereas free PhLP migrated with the tracking dye. (C) Increasing amounts of PhLP-myc immunoprecipitates identical to those of Fig. 1A were immunoblotted with antibodies specific to PhLP or TCP-1α, and band intensities were compared with standards for both PhLP and CCT to determine the stoichiometry of the coimmunoprecipitation. The average ratio of TCP-1α to PhLP from three separate immunoprecipitations was 2.2 ± 0.3 to 1. (D) CCT was immunoprecipitated from unmodified CHO cells by using the anti-TCP-1α antibody. The resulting immunoprecipitate was immunoblotted with anti-PhLP or anti-TCP-1α antibodies as indicated.