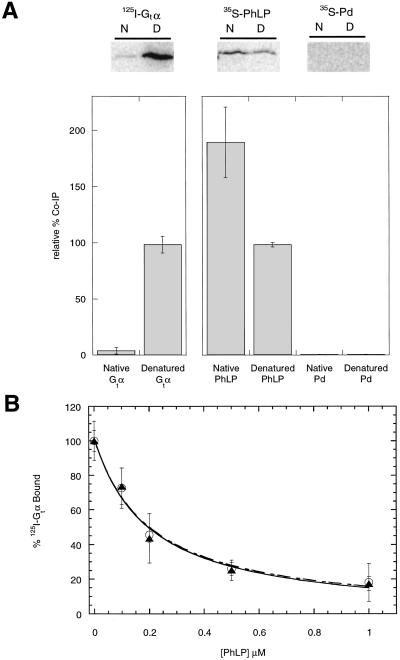
Figure 4.
Native PhLP binds CCT. (A) 125I-Gtα, 35S-PhLP, and 35S-Pd at a final concentration of 5 μM were denatured in 6 M urea or maintained in their native state in buffer without urea and then diluted 100-fold in the presence of CCT from rabbit reticulocyte lysates. Samples were immunoprecipitated by using the anti-TCP-1α antibody and subjected to SDS/PAGE and phosphorimaging. N and D indicate respectively native or urea-denatured samples. The value of urea-treated Gtα was set at 100% for the 125I-labeled samples and the value of urea-treated PhLP was set at 100% for the 35S-labeled samples. Error bars represent the standard deviation from three separate experiments. (B) PhLP was denatured under the same conditions as above. Urea-treated (○) or nontreated (▴) PhLP samples were then incubated with 0.2 μM 125I-Gtα, 0.2 μM Gtβγ, and urea-stripped retinal rod outer segment membranes (1.0 μM rhodopsin). The binding of Gt to light-activated rhodopsin was then assayed as described (15). The final dilution of urea had no effect on the total binding. Error bars represent the standard deviation from three separate experiments.