Abstract
Background
Heat stress (HS) is a significant challenge in poultry, negatively impacting feed efficiency and survival. These adaptive responses could lead to disrupted lipid metabolism, impaired immunity, and neural damage. We hypothesized that the neuroendocrine system plays a central role in HS responses by activating the hypothalamic-pituitary-adrenal and sympathetic-adrenal-medullary axes. This study explores the neural transcriptomic changes and metabolomic profile subjected to HS, aiming to unravel the complexity of nervous adaptive responses, including the disrupted lipid metabolism, impaired immunity, and neural damage processes.
Results
Based on the designed chicken HS model, we identified 1,871 serum metabolites, with 149 differentially accumulated metabolites (DAMs), including ursodeoxycholic acid, niacinamide, and prostaglandin E1. These metabolites indicated disrupted lipid homeostasis and potential adaptive mechanisms to mitigate stress. Key metabolite biomarkers were identified using random forest, including 2-(Methylthio)phenol, and Deca-2,4,6-triynedioic acid, enriched in immune and stress-related pathways such as glutathione metabolism and nicotinamide metabolism. Then, the comparative transcriptome analysis of the hypothalamus and pituitary revealed 358 differentially expressed genes (DEGs), highlighting tissue-specific adaptive pattern. The hypothalamus DEGs enriched in neurotransmitter biosynthesis and Notch signaling pathways, while the pituitary DEGs exhibited changes in calcium and insulin signaling pathways. Finally, by integrating omics data with machine learning, we uncovered key stress-relief mechanisms involving adenosine accumulation and adrenaline signaling. Adenosine signaling emerged as a critical pathway for thermoregulation through vascular relaxation, while adrenaline signaling regulated calcium homeostasis and muscle contraction under stress.
Conclusions
This study provides comprehensive insights into the molecular adaptations of HS in broiler, emphasizing the roles of lipid metabolism, neuroendocrine regulation, and vascular dynamics. The findings highlight the potential of integrating transcriptomics and metabolomics with machine learning to identify biomarkers and pathways involved in HS adaptation. These results could provide an effective theoretical basis of the preventive and treatment measures for HS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-025-11881-7.
Keywords: Biomarkers, Heat stress, Metabolomics and transcriptomics, Neuroendocrine regulation
Background
The poultry industry is globally essential to food security and economic stability. However, with unpredictable climate patterns, managing heat stress (HS) has become a challenge, as it can reduce growth rates, feed efficiency, and survival rate [1, 2]. As a consequence, there is a deepening research focus on the biological underpinnings of how HS affects broilers [3], for example, heat shock proteins (HSPs) [4, 5], xanthine dehydrogenase (XDH), and periostin (POSTN) [6] are abundant biomarker proteins activated in response to HS. While recent studies have increasingly focused on the complex responses of blood metabolites and neural tissue as indicators of adaptive or maladaptive physiological changes [7, 8].
The neuroendocrine system (NES) played critical role in HS responses. HS activates the sympathetic-adrenal-medullary (SAM) axis and the hypothalamic-pituitary-adrenal (HPA) axis. The SAM axis releases adrenaline, leading to tachycardia and increased respiratory rates in chickens, which rapidly accelerates glucose synthesis [9]. Conversely, the HPA axis releases corticosterone more slowly than adrenaline but with longer-lasting effects. Consequently, corticosterone is widely recognized as a biomarker of stress. Elevated corticosteroid levels during HS can cause hypercholesterolemia, cardiovascular issues, gastrointestinal lesions, and impaired immune function [10]. Vasodilation, excessive sweating, and rapid panting increase blood pH, resulting in respiratory alkalosis. This disrupts bicarbonate and ionized calcium balance, impairing egg production [11]. Specifically, whole egg weight, eggshell thickness, weight, and strength decrease by 3.24%, 1.2%, 9.93%, and 0.66%, respectively [12]. The prolonged HS decreases feed intake by 16.4%, reduces body weight by 32.6%, and increases feed conversion ratio by 25.6% in broilers at 42 days age [13]. Chronic HS disrupts lipid metabolism and lowers muscle protein content [14]. High temperatures affect broiler production via cecal microbiota and their metabolites, mediated by the glucose-sensing receptor–gut peptide–glucolipid metabolism axis [15, 16]. In parallel, HS responses are primarily managed by the hypothalamus and pituitary, which integrate stress signals to regulate physiological and behavioral adaptations to mitigate HS [17, 18].
The thermoreceptors were primarily found in hypothalamus, eliciting responses similar to those of cutaneous thermoreceptor signals [18, 19]. These hypothalamic signaling provided insights into the effects of HS on the nervous system. In addition, HS can lead to substantial damage to organs, such as acute degeneration of myocardial cells [20], inflammatory cell infiltration in the liver [21], and liver injury due to increased autophagy via the Nrf2-Keap1 signaling pathway [22, 23]. In light of these intricate biological interactions, traditional analytical methods are often insufficient to capture the full scope of interaction network.
Recently, machine learning (ML) methods are increasingly adapted in biomarker discovery because of their substantial promise in handling multi-omics datasets and identifying complex interactions [24, 25]. A recent study integrating non-targeted metabolomics with random forest (RF) machine learning analysis identified d-xylose, xanthine, and pyruvaldehyde as potential key marker compounds linked to freshness deterioration in chilled pork [26]. In this study, we employed non-targeted metabolome approach, identifying 1,871 metabolites across HS and control cohort of chronic heat stress in white-feather broiler. We apply RF algorithm to identify hub-elements comprising nervous DEGs and key serum metabolites, and reveal interaction networks that adenosine accumulation and adrenaline signaling. These findings offer profound insights into the metabolomic and neural transcriptomic adaptations underlying HS responses in chickens.
Result
Identification of metabolites accumulated in the HS
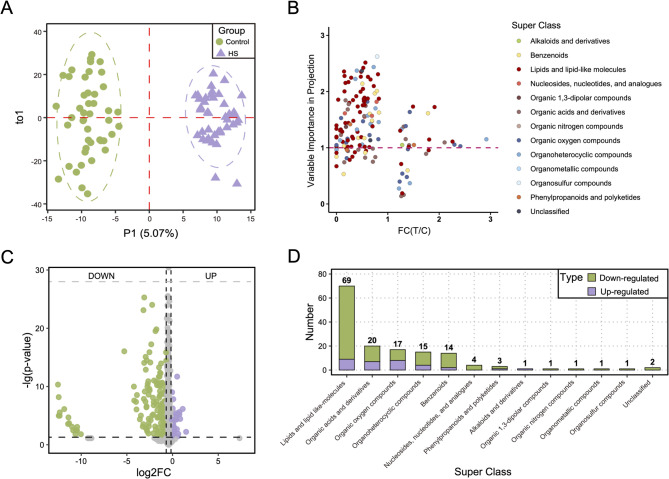
A total of 1,871 metabolites were detected in serum samples from 86 chicks (Control: n = 45; HS: n = 41). The largest category was lipids and lipid-like molecules, comprising 597 metabolites (31.9%), followed by organic acids and derivatives (393 metabolites, 21.0%), and Organoheterocyclic compounds (285 metabolites, 15.2%). Organic oxygen compounds constituted 161 metabolites (8.6%), while benzenoids also contributed 152 metabolites (8.1%). Other notable groups included phenylpropanoids and polyketides with 89 metabolites (4.8%), and nucleosides, nucleotides, and analogues, comprising 51 metabolites (2.7%). A small fraction of metabolites (56 compounds, 3.0%) remained unclassified, potentially due to limitations in current annotation databases (Additional file 1: Table S1, Fig. 1a). In addition, categories with less than 50 metabolites are uniformly classified into the Other category (Fig. 1b).
Fig. 1.
Categories, numbers and proportions of annotated metabolites. Super class with fewer than 50 metabolites are grouped into the Other category. A, Taxonomic categories of annotated metabolites. B, Categories, numbers and proportions of other class metabolites
The OPLS-DA analysis for metabolites indicated a significant separation between HS and control group (Fig. 2a), where the R2Y (96%) and Q2Y (80%) of this model were both nearly 1, reflecting strong model performance. The permutation test results demonstrated that the R2Y and Q2Y values of randomized models (scatter points) were consistently lower than those of the original model (solid line), suggesting that the original model exhibits robust predictive ability (Additional file 2: Figure S1). Among these metabolites, 149 DAMs were identified between the HS and control groups (Fig. 2c; Additional file 3: Table S2). Of these, 117 metabolites (78.5%) were significantly reduced in the HS group compared to controls (e.g., ursocholic acid and niacinamide), while 32 metabolites (21.5%) were elevated in the HS group (e.g., prostaglandin E1 (PGE1), polidocanol, and 3-decenoylcarnitine). The details of the top 50 DAMs is provided in Additional file 4: Figure S2. Ursocholic acid, an intermediate in bile acid breakdown, generated secondary bile acids, which regulated lipid and glucose metabolism, modulate liver inflammation, and support productivity, gut health, heat stress relief, and liver protection [27]. Prostaglandin E1 is known to regulates blood pressure and vascular dilation and can alleviate AngII-induced cardiac hypertrophy by activating EP3 receptors and upregulating Ntn1 [28]. Lipids and lipid-like molecules were the most abundant differential metabolites (69 compounds, 46%), followed by organic acids and derivatives (13%) and organic oxygen compounds (11%) (Fig. 2d; Additional file 5: Table S3). We observed that the result of OPLS-DA analysis was consistent with DAMs, where 77.8% DAMs with VIP > 1 (Fig. 2b).
Fig. 2.
Identification and characterization of differentially accumulated metabolites (DAMs) between heat stress (HS) and control groups. A OPLS-DA of untargeted metabolomics data comparing HS group (colored in purple) and control group (colored in green). B Distribution of variable importance in projection (VIP) values for DAMs. C Volcano plot of the detected metabolites between the HS group and control group. DAMs are colored in purple (upregulated) and green (downregulated). D Histogram of number distributions of DAMs in super class: upregulated (purple), downregulated (green)
Machine learning-based selection of signature metabolites
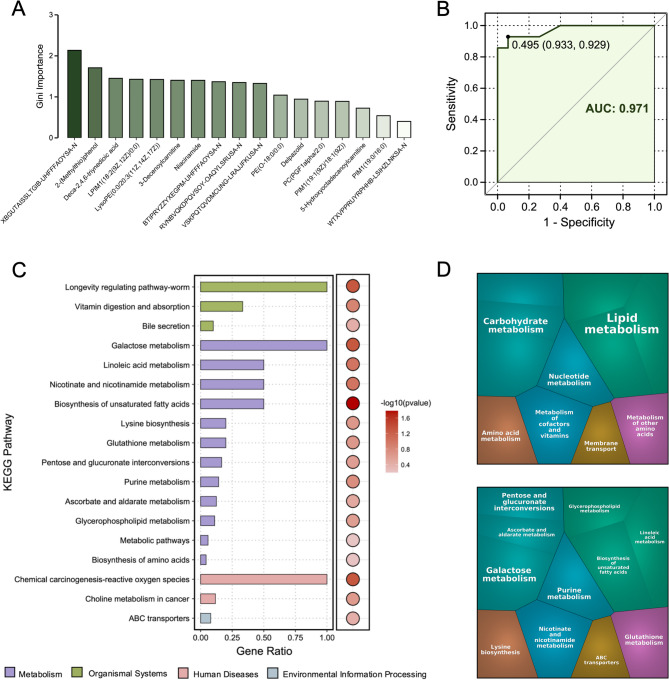
Here, we trained a RF model to screen metabolite markers based on DAMs with VIP > 1. Based on the average rank and cross-validation error, the top classification RF (100 trees) recognized 17 metabolites as feature indicators, indicating excellent classification performance (AUC = 0.97) (Fig. 3b). Among these, XBGUTAISSLTGIB-UHFFFAOYSA-N, 2-(Methylthio)phenol, and Deca-2,4,6-triynedioic acid were considered most significant (Fig. 3a).
Fig. 3.
Selection of characteristic metabolites and KEGG pathway enrichment analysis. A Histogram of Gini importance distribution for characteristic metabolites. B Receiver operating characteristic (ROC) of the Random Forest (RF) model. C KEGG pathway enrichment analysis of metabolites. The bar chart represents the gene ratio for the enrichment analysis, while the bubble indicates the significance of the metabolite enrichment, with darker colors denoting higher significance. D Proteomaps analysis of KEGG pathway enrichment significance
These metabolites were mapped to the 11 immune and stress related KEGG metabolic pathways (Fig. 3c; Additional file 6: Table S4), including glutathione metabolism, ascorbate, aldarate metabolism, nicotinate, and nicotinamide metabolism (Fig. 3d). Glutathione, a key antioxidant, is known to protected sulfhydryl-containing proteins and enzymes in cell membranes from oxidation [29]. Nicotinate and nicotinamide, both forms of vitamin B3, can be interconvertible in metabolic pathways. Nicotinamide minimizes reactive oxygen species and free radicals, shielding cells from oxidative damage and maintaining integrity [30].
Functional enrichment analysis of the neural transcriptomic adaptations underlying HS responses in chickens
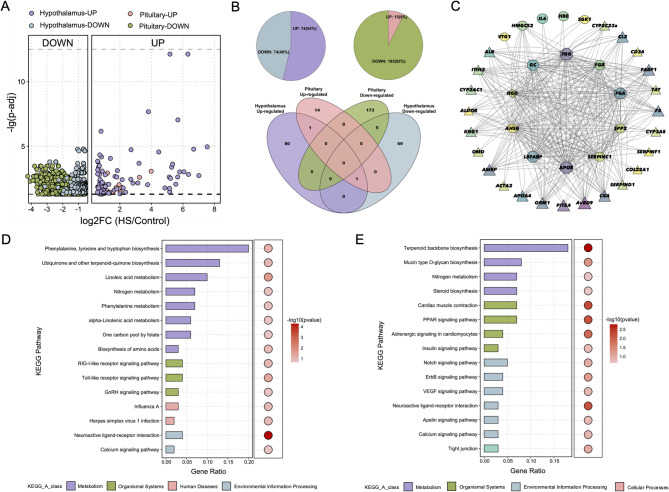
To investigate the molecular basis of neural tissue of the metabolic differences detected in HS and control group, transcriptome sequencing was performed on pituitary (Control: n = 18; HS: n = 19) and hypothalamus (Control: n = 19; HS: n = 19) tissues. Comparative transcriptomic analysis between HS and control groups revealed significant differential gene expression patterns in both hypothalamic and pituitary tissues (Fig. 4a). In the hypothalamus, we identified 160 differentially expressed genes (DEGs), consisting of 74 genes significantly upregulated and 86 genes downregulated in HS group relative to controls (Additional file 7: Figure S3a; Additional file 8: Table S5). The pituitary exhibited an even more pronounced response, with 198 DEGs detected, of which 183 genes showed increased expression while only 15 genes were suppressed under heat stress conditions (Additional file 7: Figure S3b; Additional file 9: Table S6). Notably, five genes (ENSGALG00000020895, glutamate metabotropic receptor 2 (GRM2), nucleoredoxin-like 2 (NXNL2), G protein-coupled receptor 183 (GPR183), and DExH-box helicase 58 (DHX58)) were upregulated in the hypothalamus but downregulated in the pituitary. Additionally, the Homocysteine inducible ER protein with ubiquitin like domain 1 (HERPUD1) gene exhibited upregulated expression in both the hypothalamus and pituitary(Fig. 4b).
Fig. 4.
Transcriptome analysis between the HS group and control group. A the distribution of fold change (FC) values and p-adjust for differentially expressed genes (DEGs) in the hypothalamus and pituitary. B The comparison of the number of DEGs in the hypothalamus and pituitary. The pie chart represents the proportion of upregulated and downregulated genes in the hypothalamus and pituitary. The venn diagram illustrates the overlap of upregulated genes and downregulated genes between the hypothalamus and pituitary. C The interaction network of the Top 10 hub genes. Triangles refer to upregulated genes, and circles refer to downregulated genes. D The 15 most significantly enriched pathways for DEGs in the hypothalamus. The bar chart represents the gene ratio from the enrichment analysis, while the bubble chart illustrates the significance level of the enrichment analysis. E The 15 most significantly enriched pathways for DEGs in the pituitary. The bar chart represents the gene ratio from the enrichment analysis, while the bubble chart illustrates the significance level of the enrichment analysis
The protein-protein interactions among DEGs were predicted using the STRING database and analyzed for network topology using the CytoHubba plugin in Cytoscape [31]. The interaction network comprised 168 genes with a total of 910 interactions, and the topological parameters for each node (Additional file 10: Figure S4; Additional file 11: Table S7). The interactions network of top 10 genes with the highest Maximum Clique Centrality (MCC) identified that the fibrinogen alpha chain (FGA) gene as the core gene, which enhanced immune responses through innate immunity and T cell-mediated pathways(Fig. 4c) [32].
Functional enrichment analysis of DEGs in neural tissues highlighted distinct biological roles for the hypothalamus and pituitary tissues (Fig. 4d-e, Additional file 12: Figure S5, Additional file 13–14: Table S8−9). DEGs in the hypothalamus were enriched in the Phenylalanine, Tyrosine, and Tryptophan metabolism pathways, suggesting an association with the generating of neurotransmitters like dopamine, norepinephrine, and serotonin, which were known to serve as pivotal modulators of basic metabolic processes [33, 34]. The Notch signaling pathway was highly enriched, indicating a potential role for the hypothalamus’s involvement in neural adaptability [35, 36]. In contrast, DEGs in the pituitary were enriched in calcium signaling and insulin signaling pathways, which were essential for hormone release, metabolic regulation, and response to external signals [37]. The insulin signaling pathway further emphasizes the pituitary’s role in maintaining metabolic balance by regulating cell growth, hormone secretion, and metabolic processes [38]. Additionally, the enrichment of glutathione metabolism in the pituitary suggests a protective role against HS, ensuring proper function under high metabolic demands [39].
Characterization of key genes involved in adenosine accumulation induced by HS
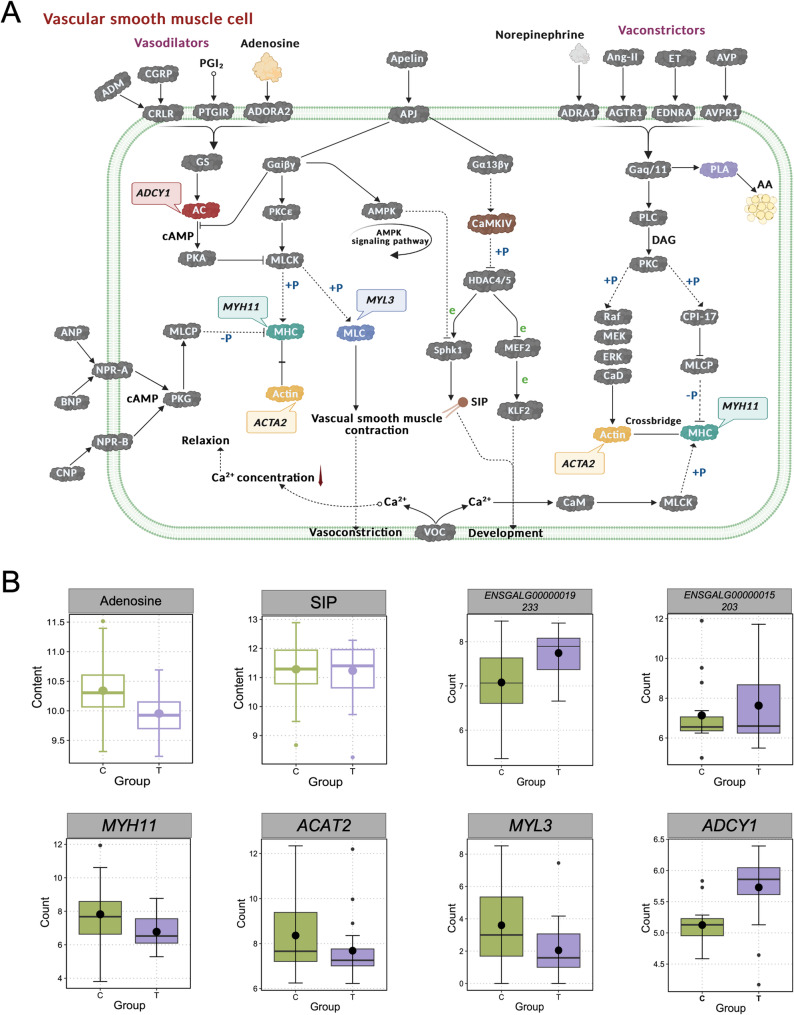
Based on metabolite markers between HS and control group, comparative transcriptome analysis of two tissues, and function enrichment analysis, we constructed stress-relieving networks of adenosine accumulation induced by HS (Fig. 5a). During heat stress, vasodilation occurred alongside increased blood flow, facilitating heat dissipation to maintain normal body temperature. The vascular tone regulation involves multiple factors, including adenosine-mediated signaling. Adenosine binds to adenosine A2 receptors (ADORA2), which activated G protein-coupled signaling pathways. The stimulatory G protein (Gs) enhances adenylate cyclase (AC) activity, encoded by the ADCY1 gene (p-adjust < 0.05, upregulated 1.12-fold) (Fig. 5b), thereby increasing cyclic adenosine monophosphate (cAMP) production. Elevated cAMP activates protein kinase A (PKA), which phosphorylates and inactivates myosin light chain kinase (MLCK) [40].
Fig. 5.
Analysis of key genes associated with adenosine accumulation induced by heat stress (HS). A The regulatory pathways of vascular contraction and relaxation (created with biorender). B Boxplot of relevant genes (ENSGALG00000019233, ENSGALG00000015203, MYH11, ACAT2, MYL3, ADCY1) and metabolites (Adenosine, Sphingosine 1-phosphate (SIP)). In the figure, the green box plot represents the Control group (Control, C), and the purple box plot represents the heat stress group (HS, T)
This inhibition reduced the phosphorylation of myosin light chains (MLC), encoded by the MYL3 (Myosin, light chain 3) gene (p-adjust < 0.05, downregulated by 0.57-fold)(Fig. 5b), and attenuated the interaction between myosin heavy chains (MHC, encoded by the MYH11 gene) and actin filaments. Consequently, vascular smooth muscle relaxed, promoting vasodilation and contributing to thermoregulation during HS [41].
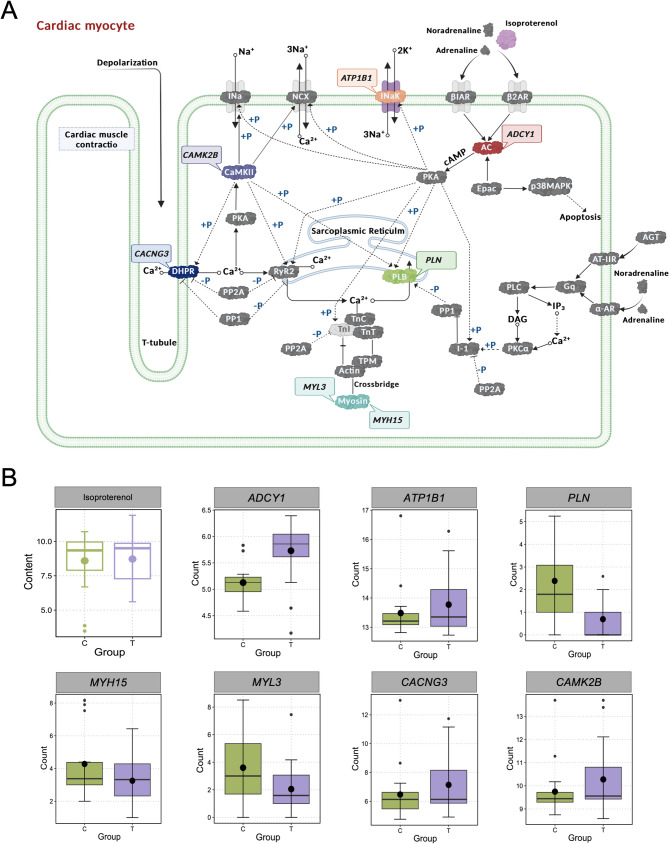
HS-induced metabolite action pathways of adrenaline signaling
HS activated the HPA and SAM axes [17], leading to a marked increase in the secretion of glucocorticoids, adrenaline, and isoproterenol (Fig. 6a). Adrenaline play a central role in modulating muscle contraction and relaxation through various signaling pathways [42], aiding poultry in coping with acute stress conditions.
Fig. 6.
Metabolic pathways of adrenaline signaling induced by heat stress (HS). A Mechanism of Action of Adrenaline(created with biorender). B, Boxplot of relevant genes (ADCY1, ATP1B1, PLN, MYH15, MYL3, CACNG3, CAMK2B) and metabolites (Isoproterenol). In the figure, the green box plot represents the Control group (Control, C), and the purple box plot represents the heat stress group (HS, T)
Specifically, adrenaline binds to β₁ and β₂ adrenergic receptors, thereby activating adenylate cyclase (AC). This enzyme converts ATP to cyclic adenosine monophosphate (cAMP), subsequently elevating intracellular calcium concentrations via downstream signaling [43]. The expression of myosin-related genes was significantly altered under stress conditions. MYH15 (Myosin, heavy chain 15) (p-adjust < 0.05; 0.76-fold down regulation; Fig. 6b) and MYL3 (p-adjust < 0.05; 0.57-fold down regulation; Fig. 6b) encode the heavy and light chains of myosin, respectively, both of which are essential structural components of the contractile apparatus.
Concurrently, the calcium regulatory network was perturbed: the phospholamban (PLN) gene (p-adjust < 0.05, downregulated by 0.29-fold) (Fig. 6b) encoded phosphorylated phospholamban (PLB), a sarcoplasmic reticulum protein that controls calcium reuptake and release by modulating SERCA (sarco/endoplasmic reticulum Ca²⁺-ATPase) activity [44]. In contrast, CACNG3 (p-adjust < 0.05, upregulated by 1.10-fold) (Fig. 6b) encoded the γ subunit of dihydropyridine receptor (DHPR), a voltage-gated calcium channel critical for excitation-contraction coupling in muscle cells. Furthermore, ATP1B1 (p-adjust < 0.05, upregulated by 1.02-fold) (Fig. 6b) encoded the β-subunit of sodium-potassium ATPase (INaK), which maintains cardiomyocyte ion homeostasis by actively transporting sodium and potassium ions across the membrane [45].
Discussion
HS undeniably impacts broiler growth and physiological function in ways that are as multifaceted as they are concerning, particularly when viewed in the larger context of global warming, which causes economic losses and welfare concerns in poultry production. The stress response mechanisms in broilers, especially the activation of the SAM and HPA axes, with adrenaline rapidly inducing effects like tachycardia and increased respiratory rates [17]. Although corticosterone can lead to more insidious outcomes such as hypercholesterolemia, cardiovascular stress, gastrointestinal distress, and immune suppression [42]. In these processes, the hypothalamus and pituitary gland, are central players orchestrating these responses, and our comparative analysis between stressed and non-stressed groups uncovered a series of dramatic shifts in gene expression profiles of the nervous system and the serum metabolite profiles. Specifically, we identified 149 DAMs and 358 DEGs across tissues. Integration with ML model revealed their intricate connections. Our analysis specifically highlighted metabolites associated with important biological pathways, particularly those involved in immune response and stress adaptation, such as glutathione metabolism and nicotinate/nicotinamide metabolism.
For metabolites accumulation, we observed both key indicators of lipid homeostasis, free fatty acids and cholesterol, showed a marked increase, further highlighting how HS disrupts lipid metabolism—an observation that aligns well with earlier studies demonstrating similar patterns of lipid metabolism disorder in heat-stressed broilers [46]. Interestingly, OPLS-DA analysis validated these DAMs, such as ursodeoxycholic acid, niacinamide, and prostaglandin E1, which not only play critical roles in lipid regulation [47], but also are implicated in blood pressure control and the alleviation of stress-related damage [48]. Prostaglandin E1, in particular, was previously noted by Hii et al. to ameliorate intestinal and systemic inflammation as well as multi-organ dysfunction in animals under HS, further underscoring its relevance here [49]. Alongside these insights, ML models have been increasingly utilized for metabolite prioritization, which effectively narrow down the vast pool of potential metabolites, enhancing the efficiency and accuracy of marker metabolites detection [50, 51]. Metabolites like 2-mercaptophenol, nicotinamide, and nigellidine emerged as key players, all closely tied to the intricate mechanisms underlying HS adaptation [29, 52].
The 3-hour post-heat stress (HS) sampling timepoint was selected based on its alignment with the widely adopted 2–4 h acute HS exposure paradigm established in prior research [53, 54]. Substantial evidence indicates that stress-responsive genes, including heat shock transcription factor 1 (HSF1) [55–57], nuclear receptor subfamily 3 group C member 1 (NR3C1) [58, 59], and corticotropin releasing hormone (CRH) [60, 61] gene, exhibit peak expression dynamics synchronously with cortisol surges and chromatin remodeling at glucocorticoid receptor-binding sites. Although this experimental design effectively captures early-phase transcriptional adaptations such as Notch signaling activation and neurotransmitter biosynthesis, it presents inherent limitations in resolving delayed metabolic feedback mechanisms like nicotinamide salvage pathways [62]. Furthermore, the single timepoint approach impedes differentiation between transient compensatory responses (e.g., acute cholesterol elevation) and pathological dysregulation. Future investigations should implement serial sampling protocols (0.5 h, 3 h, 6 h) to elucidate kinetic relationships within adenosine signaling pathways and vascular adaptation processes, while maintaining the 3-hour benchmark to facilitate cross-study comparisons.
The observed differential expression patterns of key genes in the hypothalamus and pituitary under heat stress provide valuable insights into the potential molecular mechanisms underlying the neuroendocrine response to thermal challenge. Notably, the contrasting regulation of GRM2, NXNL2, GPR183, and DHX58 between the hypothalamus (upregulated) and pituitary (downregulated) suggests tissue-specific roles in mediating heat stress adaptation. The upregulation of GRM2 (glutamate metabotropic receptor 2) gene in the hypothalamus aligns with its role as a modulator of excitatory neurotransmission. Glutamate signaling is critical for stress-responsive pathways [63], and increased GRM2 expression may reflect heightened neuronal activity to coordinate systemic responses to heat stress. Conversely, its down regulation in the pituitary could indicate a feedback mechanism to attenuate excessive stress hormone release [64], such as ACTH or prolactin, to prevent overactivation of the hypothalamic-pituitary-adrenal (HPA) axis [65]. The consistent up regulation of HERPUD1 in both tissues underscores its critical role in endoplasmic reticulum (ER) stress response [66, 67]. Heat stress likely initiates protein misfolding cascades, with HERPUD1 activation serving as a regulatory node that coordinates the unfolded protein response (UPR) to reestablish proteostatic equilibrium [68]. Mechanistically, this proteostatic mechanism is directly governed by the transcription factor Nrf1 through its binding to antioxidant response elements in the HERPUD1 promoter—a regulatory relationship evidenced by abolished HERPUD1 induction in Nrf1 knockout cells [69]. This ubiquitous upregulation suggests ER stress as a central feature of heat stress pathophysiology across neuroendocrine tissues.
Hypothalamic DEGs were highly enriched in pathways related to neurotransmitter synthesis (e.g., glutamate and GABA pathways) and neural plasticity, underscoring the hypothalamus’ central role in orchestrating neuroendocrine responses to physiological and environmental stressors [18, 19]. Meanwhile, pituitary DEGs highlighted its involvement in calcium and insulin signaling pathways, suggesting its pivotal role in maintaining metabolic homeostasis through hormone secretion [70], a link further evidenced by the up regulation of NF-κB signaling genes, which hints at the activation of inflammatory responses in the context of HS [71]. Furthermore, genes tied to lipid metabolism, such as PPARα, showed altered expression patterns that echoed the metabolomic data, adding layer of corroboration to the story of disrupted lipid regulation [46]. However, while these tissue-specific findings elucidate HPA axis engagement, the cross-tissue signaling networks connecting peripheral organs (e.g., gut, liver) to central neuroendocrine regulation remain unresolved. Emerging evidence suggests that peripheral metabolites may coordinate systemic adaptation to heat stress through interorgan signaling, though the precise molecular conduits remain uncharacterized. For instance, gut-derived ursodeoxycholic acid (UDCA), which is significantly elevated under heat stress, potentially crosses the blood-brain barrier via organic anion transporters (e.g., OATP1A4 [72]) and binds to the farnesoid X receptor (FXR) in the hypothalamus [73, 74], thereby modulating the expression of glucocorticoid sensitivity-related genes (e.g., NR3C1) [75]. This mechanism aligns with hepatic observations, where UDCA suppresses CYP7A1 expression to mitigate cholesterol accumulation, synchronously alleviating lipid metabolic dysregulation in both central and peripheral tissues [76]. Another key metabolite, PGE1, may exhibit dual regulatory roles: (1) activating hypothalamic EP3 receptors to stimulate corticotropin-releasing hormone (CRH) release [28, 77], and (2) enhancing hepatic gluconeogenesis via the cAMP-PKA pathway to fuel thermoregulatory energy demands [78, 79]. Furthermore, nicotinamide trafficking across tissues may integrate metabolic and epigenetic regulation—the hypothalamus salvages nicotinamide via nicotinamide phosphoribosyltransferase (NAMPT) to sustain NAD + synthesis for hormonal stability [80], while the heart methylates excess nicotinamide via nicotinamide N-methyltransferase (NNMT) [81], providing substrates for hypothalamic CRH gene demethylation [82]. Although these mechanisms require further validation, they suggest that metabolites serve as molecular bridges, translating environmental stress signals into epigenetic and metabolic responses. Future studies could employ spatially resolved transcriptomics to map metabolite gradients within vascular networks, coupled with conditional knockout models targeting gut-liver-brain signaling nodes to dissect multi-organ crosstalk mechanisms.
The vascular adaptations during HS also deserve attention, as vasodilation and enhanced blood flow are vital for dissipating heat and stabilizing body temperature, mechanisms strongly influenced by adenosine signaling [83]. This pathway mediates vasodilation through activation of adenosine A2 receptors and associated G protein-coupled signaling [84], as highlighted by both our findings and prior studies like Ritchie et al., which explored how caffeine, an adenosine receptor antagonist, altered thermoregulation under heat stress [85]. Building on this, our exploration of adrenaline signaling pathways revealed nuanced regulation of genes such as MYH15, PLN, CACNG3, and ATP1B1, all of which play roles in muscle contraction, calcium handling, and ion homeostasis.
These results align well with existing research, showing how β-adrenergic signaling governs calcium dynamics, including the phosphorylation of skeletal muscle CaV1.1 channels to boost calcium influx during stress, a mechanism critical for muscle contractility [86]. The role of phospholamban, too, emerged as central; its regulation of the SERCA pump was shown to impact calcium reuptake, influencing muscle strength and recovery [87]. Altogether, these results shed light on the complex, interconnected processes that underpin the adaptation of broilers to HS, encompassing neuroendocrine control, metabolite accumulation, and transcriptomic shifts.
Conclusions
This study systematically investigates the physiological and molecular adaptations of broilers to HS, integrating metabolomic and transcriptomic profiling. HS disrupts lipid metabolism, as shown by key metabolites like ursodeoxycholic acid and niacinamide, which aid stress mitigation and vascular stability. These metabolites are functionally implicated in stress adaptation and vascular function regulation through their antioxidant and anti-inflammatory activities. Transcriptomic analysis highlights the roles of the hypothalamus in neurotransmitter production and the pituitary in hormone regulation and metabolic balance. Mechanistic insights reveal adenosine signaling supports thermoregulation via vasodilation, while adrenaline signaling maintains calcium homeostasis and muscle function. Machine learning identified biomarkers such as 2-(Methylthio) phenol and Deca-2,4,6-triynedioic acid, linked to glutathione and nicotinamide metabolism pathways, offering strategies to mitigate HS. These findings reveal the specific mechanisms by which heat stress affects the neuroendocrine system in poultry, highlighting the interconnections between neuroendocrine axes and organ damage. The molecular signatures and pathway interactions identified in this study propose testable targets for dietary intervention trials, particularly through modulation of nicotinamide salvage pathways and adenosine receptor signaling, pending validation in controlled feeding studies.
Materials and methods
Chick materials and sampling
All chickens were obtained from the fast-growing white-feathered pure line, produced by Xinguang Agricultural and Animal Industrials Co., Ltd. (Mile, China). The 86 healthy broilers at 42 days, raised under standard environmental and nutritional conditions (NY/T 33-2004), were randomly divided into a HS group (41 chicks) and a control group (45 chicks). While, the HS was induced by raising the lab temperature from 24℃ to 38 ℃ within 0.5 h and continued to be treated for 3 h [88]. The temperature monitoring showed at Additional file 15: Tables S10. After the treatment, blood samples from the wing veins of all individuals were collected (serum was isolated for metabolomics sequencing). In this study, all experimental subjects were anesthetized via intramuscular injection of ketamine hydrochloride (50 mg/mL) at 60 mg/kg body weight. Surgical anesthesia depth was confirmed by loss of palpebral reflex and absence of corneal reflexes. Following achievement of unconsciousness, broilers were euthanized by cervical dislocation. Following the standard of the Poultry Performance Terminology and Poultry Determination (China, NY/T 823–2020), pituitary and hypothalamus tissues were obtained for subsequent RNA extraction.
Metabolite extraction
The serum samples were pre-processed before metabolomics sequencing (Additional file 16: Table S11). Here, we adapted non-targeted metabolomics sequencing using an ultra-performance liquid chromatography-tandem mass spectrometry system. Separation was achieved with a C18 column (1.8 μm, 2.1 mm × 100 mm). The mobile phases were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), with a flow rate of 0.35 mL/min, column temperature at 45 °C, and injection volume of 2 µL. Samples were kept at 4 °C during analysis. After separation, the samples were analyzed using high-resolution ion mobility mass spectrometry (parameters listed in Additional file 17: Table S12). Data were collected in full-scan mode (m/z 50–1000) with MSE, alternating between low-energy (CE 4 eV) and high-energy (CE 20–45 eV) scans for ion fragmentation. Argon (99.99%) served as the collision gas. The quality control samples were prepared by mixing equal volumes of extraction solutions from all individual samples. The correlation of the quality control samples was 0.99 (Additional file 18: Figure S6), indicating stable instrument operation and reliable results. To ensure data reliability, four internal standards were employed: [²H₄]-succinic acid (succinic acid-D4), L-2-chlorophenylalanine, D-luciferin free acid, and [²H₄]-cholic acid (cholic acid-D4). These standards were selected to cover diverse chemical classes (organic acids, amino acid analogues, and bile acids), thereby monitoring extraction efficiency and instrumental performance across metabolite categories. The relative standard deviations (RSDs) of all internal standards were below 30%, indicating stable instrument response. Additionally, the retention time shifts of these standards across all samples were confined to ± 18 s, further demonstrating the robustness of the chromatographic method. These metrics collectively confirm the stability of the analytical platform and the reliability of the detection process. While the raw LC-MS data were processed using Progenesis QI V2.3 for baseline filtering, peak detection, alignment, and normalization (key parameters: 5 ppm precursor tolerance, 10 ppm product tolerance, 5% ion threshold). Compounds were identified using databases (HMDB, LipidMaps, Metlin, etc.) based on charge-mass ratio, fragments, and isotopes. Peaks with > 50% missing values were removed, missing data were imputed with half the minimum value, and positive/negative ion data were merged into a single matrix.
Identification of signature metabolites accumulated in HS and control cohort
Using A two-tailed Student’s t-test to determine the significance of metabolites’ differences between groups. The Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) was performed to rank each metabolites’ contribution (variable importance in projection, VIP) to phenotypic discrimination. Differentially accumulated metabolites (DAMs) were identified by comparing metabolite abundance between HS (n = 41) and control (n = 45) groups, with FC calculated as HS/Control. The screening criteria such as p-value < 0.05,|log2(FC)| ≥ 0.26 were applied.
Random Forest is an efficient feature selection technique and has shown promising applications in metabolome data analysis [89]. We used the RF function of caret package in R (v4.3.2). The Random Forest model was implemented with 100 decision trees. The feature selection process employed all available metabolomic data to ensure a comprehensive assessment of potential candidates. Following feature selection, the dataset was partitioned into training (65%) and validation (35%) subsets using stratified random sampling to maintain balanced class distributions. The predictive model was then constructed using the selected features, and to enhance the model’s robustness, we employed 10-fold cross-validation during the training phase. This process involved dividing the training data into ten subsets, training the model on nine of these subsets, and validating it on the remaining subset. This procedure was repeated ten times, ensuring that each data point was used for both training and validation. Feature importance was quantified using permutation importance (z-scores) derived from 1000 Monte Carlo permutations, with significance assessed at p-value < 0.05 based on null distributions generated through permutation. Model performance was rigorously evaluated on the validation set, with classification performance quantitatively assessed by calculating the area under the receiver operating characteristic curve (AUC). The selection was guided by the significance of these metabolites in classification, accounting for variations across metabolome samples. Next, we deduced the promising metabolites that overlapped among DAMs, metabolites with VIP ≥ 1 and the featured metabolites.
mRNA sequencing and differentially expressed genes
Total RNA extraction, transcriptome libraries construct, and paired end sequencing were consistent with previous research [90]. Total RNA was extracted using TRIzol reagent according to the instructions, the purity and quantification of RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA), and the RNA integrity was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) (Additional file 19: Table S13). Transcriptome libraries were constructed using the VAHTS Universal V6 RNA-Seq Library Prep Kit according to the instructions. The libraries were sequenced using the Illumina NovaSeq 6000 sequencing platform, and 150 bp paired-end reads were generated. Approximately 50 million raw reads were obtained for each sample (Additional file 19: Table S13). Raw reads of fastq format were firstly processed using Fastp [91] and the low quality reads were removed to obtain the clean reads. Then about 47 million clean reads for each sample were retained for subsequent analyses. The clean reads were mapped to the reference genome (GRCg6a) using HISAT2 (v2.1.0) [92]. FPKM [93] of each gene was calculated and the read counts of each gene were obtained by HTSeq-count (v0.11.2). Genes with zero expression across all samples were excluded from subsequent analysis. Differentially expressed genes (DEGs) between HS and control groups were identified using the DESeq2 package in R (v4.3.2), with gene expression differences quantified by fold change (FC). The analysis compared HS samples directly against controls, using a p-adjust threshold of < 0.05 (Benjamini-Hochberg method) and an absolute|log2(fold change)| >0.59 to identify differentially expressed genes (DEGs). Then, we brined the STRING website (https://cn.string-db.org) and Cytoscape software (v 3.9.1) to construct the DEGs interaction network.
Enrichment analysis
Metabolite Enrichment Analysis was performed using the MetaboAnalyst 5.0 web-based platform (https://www.metaboanalyst.ca). Metabolites were mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathway database. Gene Functional Enrichment Analysis was conducted using the R package clusterProfiler (v4.12.1). Ensembl gene IDs were converted to NCBI Entrez Gene IDs via the Omicshare platform (https://www.omicshare.com) to ensure compatibility with annotation resources, prior to enrichment analysis with the Gallus database org.Gg.eg.db. Differentially expressed genes were annotated against Gene Ontology (GO) biological processes and KEGG pathways. GO term and KEGG pathways with p-value < 0.05 were considered significantly enriched, adjusted for multiple testing using the Benjamini-Hochberg (BH) method.
Supplementary Information
Additional file 1: Table S1. The metabolites categories
Additional file 2: Figure S1. OPLS-DA permutation test plot
Additional file 3: Table S2. The details of 149 DAMs
Additional file 4: Figure S2. Characteristic distribution of the Top 50 DAMs
Additional file 5: Table S3. The class proportion of DAMs
Additional file 6: Table S4. The enrichment details of DAMs
Additional file 7: Figure S3. Volcano plot of the differentially expression genes in hypothalamus (A) and pituitary (B)
Additional file 8: Table S5. The details of 160 hypothalamic DEGs
Additional file 9: Table S6. The details of 198 pituitary DEGs
Additional file 10: Figure S4. Interaction network of differentially expressed genes
Additional file 11: Table S7. The details of PPI network topology
Additional file 12: Figure S5. Bar plot of GO terms (Top 60) enriched for hypothalamus (A) and pituitary (B) differentially expressed genes
Additional file 13: Table S8. The enrichment details of hypothalamic DEGs
Additional file 14: Table S9. The enrichment details of pituitary DEGs.
Additional file 15: Table S10. The temperature monitoring records of HS
Additional file 16: Table S11. Pre-processing of serum samples before metabolome sequence
Additional file 17: Table S12. The gradient program of ultra-performance liquid chromatography -tandem mass spectrometry system (comprised an ultra-performance liquid chromatography system and a high-resolution mass spectrometer)
Additional file 18: Figure S6. Correlation matrix diagram of quality control samples
Additional file 19: Table S13. RNA-seq Data Production and Quality Assessment Metrics
Acknowledgements
We would like to express our gratitude to the members of Mile Xinguang Agricultural and Animal Industrials Co., Ltd. (Mile, China) for their assistance in the phenotype collection and sampling. The RNA libraries were sequenced by OE Biotech, Inc., Shanghai, China. We are grateful to OE Biotech, Inc., (Shanghai, China) for assisting in sequencing. We are also very grateful to OE Biotech, Metabolomics testing provided by Inc., Shanghai, China.
Abbreviations
- HS
Heat stress
- DAMs
Differentially accumulated metabolites
- DEGs
Differentially expressed genes
- HSPs
Heat shock proteins
- XDH
Xanthine dehydrogenase
- POSTN
Periostin
- NES
Neuroendocrine system
- SAM
Sympathetic-adrenal-medullary
- HPA
Hypothalamic-pituitary-adrenal
- ML
Machine learning
- RF
Random forest
- PGE1
Prostaglandin E1
- FC
Fold change
- OPLS-DA
Orthogonal Partial Least Squares Discriminant Analysis
- VIP
Variable importance in projection
- AUC
Area under the receiver operating characteristic curve
- ROC
Receiver operating characteristic
- GO
Gene Ontology
- BH
Benjamini-Hochberg
- GRM2
Glutamate metabotropic receptor 2
- NXNL2
Nucleoredoxin-like 2
- GPR183
G protein-coupled receptor 183
- DHX58
DExH-box helicase 58
- HERPUD1
Homocysteine inducible ER protein with ubiquitin like domain 1
- FGA
Fibrinogen alpha chain
- MCC
Maximum clique centrality
- ADORA2
Adenosine A2 receptors
- Gs
G protein
- AC
Adenylate cyclase
- cAMP
Cyclic adenosine monophosphate
- PKA
Protein kinase A
- MLCK
Myosin light chain kinase
- MLC
Myosin light chains
- MYL3
Myosin, light chain 3
- MHC
Myosin heavy chains
- MYH15
Myosin, heavy chain 15
- PLN
Phospholamban
- PLB
Phosphorylated phospholamban
- SERCA
Sarco/Endoplasmic Reticulum Ca²⁺-ATPase
- DHPR
Dihydropyridine receptor
- INaK
β-subunit of sodium-potassium ATPase
- HSF1
Heat shock transcription factor 1
- NR3C1
Nuclear receptor subfamily 3 group C member 1
- CRH
Corticotropin releasing hormone
- HPA
Hypothalamic-pituitary-adrenal
- ER
Endoplasmic reticulum
- UPR
Unfolded protein response
- UDCA
Ursodeoxycholic acid
- FXR
Farnesoid X receptor
- CRH
Corticotropin-releasing hormone
- NAMPT
Nicotinamide phosphoribosyltransferase
- NNMT
Nicotinamide N-methyltransferase
Authors’ contributions
Guiping Zhao and Jie Wen conceptualized and designed the project. Fan Ying conducted the experiments. Dawei Liu supplied the study reagents and instrumentation. Peihao Liu and Zhirui Yang carried out the bioinformatics analysis. Bingxing An and Peihao Liu drafted and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
This work was supported by funds from the Central Public-interest Scientific Institution Basal Research Found (No. 2023-YWF-ZYSQ-08) and the Key Program of the National Natural Science Foundation of China (32230101).
Data availability
The RNA-seq data generated in this study have been deposited in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under the accession number SUB14651504 and the BioProject number PRJNA1230596.The metabolomics dataset generated in this study has been submitted to the China National Center for Bioinformation (CNCB) OMIX database under the accession number OMIX009324 and the BioProject number PRJCA036939.
Declarations
Ethics approval and consent to participate
The use of all chickens has been approved by Xinguang Agricultural and Animal Industries Co., Ltd. (Mile, China). All animal procedures, including ethical and animal welfare issues, were approved by the Science Research Department of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (CAAS), Beijing, China (approval number: IAS2024-38).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bingxing An and Peihao Liu contributed equally to this work.
References
- 1.Huang Y, Cai H, Han Y, Yang P. Mechanisms of heat stress on neuroendocrine and organ damage and nutritional measures of prevention and treatment in poultry. Biology. 2024;13(11):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock Industries1. J Dairy Sci. 2003;86:E52–77. [Google Scholar]
- 3.Nawaz AH, Amoah K, Leng QY, Zheng JH, Zhang WL, Zhang L. Poultry Response to Heat Stress: Its Physiological, Metabolic, and Genetic Implications on Meat Production and Quality Including Strategies to Improve Broiler Production in a Warming World. Front Veterinary Sci. 2021;8:699081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie J, Tang L, Lu L, Zhang L, Xi L, Liu HC, Odle J, Luo X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS ONE. 2014;9(7):e102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abare MY, Rahayu S, Tugiyanti E. Review: the role of heat shock proteins in chicken: insights into stress adaptation and health. Res Vet Sci. 2023;165:105057. [DOI] [PubMed] [Google Scholar]
- 6.Liang Q, Huan S, Lin Y, Su Z, Yao X, Li C, Ji Z, Zhang X. Screening of heat stress-related biomarkers in chicken serum through label-free quantitative proteomics. Poult Sci. 2024;103(2):103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jastrebski SF, Lamont SJ, Schmidt CJ. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE. 2017;12(7):e0181900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang ZY, Jia GQ, Zuo JJ, Zhang Y, Lei J, Ren L, Feng DY. Effects of constant and Cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult Sci. 2012;91(11):2931–7. [DOI] [PubMed] [Google Scholar]
- 9.Ayo JO, Obidi JA, Rekwot PI. Effects of heat stress on the well-being, fertility, and hatchability of chickens in the Northern Guinea Savannah zone of nigeria: a review. ISRN Veterinary Sci. 2011;2011:838606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, Handa RJ. The Hypothalamic-Pituitary-Adrenal axis: development, programming actions of hormones, and Maternal-Fetal interactions. Front Behav Neurosci. 2020;14:601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Tarabany MS. Effect of thermal stress on fertility and egg quality of Japanese quail. J Therm Biol. 2016;61:38–43. [DOI] [PubMed] [Google Scholar]
- 12.Mack LA, Felver-Gant JN, Dennis RL, Cheng HW. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult Sci. 2013;92(2):285–94. [DOI] [PubMed] [Google Scholar]
- 13.Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Ijaz A, Sohail A, Shabbir MZ, Rehman H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 2012;91(9):2235–40. [DOI] [PubMed] [Google Scholar]
- 14.Kim H-W, Cramer T, Ogbeifun O, Seo J-K, Yan F, Cheng H, Kim Y. Breast meat quality and protein functionality of broilers with different probiotic levels and Cyclic heat challenge exposure. Meat Muscle Biology. 2017;1:81. [Google Scholar]
- 15.Cao C, Chowdhury VS, Cline MA, Gilbert ER. The Microbiota-Gut-Brain Axis during heat stress in chickens: A review. Front Physiol. 2021;12:752265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Li X, Zhou Y, Feng J, Zhang M. Effects of heat stress on Gut-Microbial metabolites, Gastrointestinal peptides, glycolipid metabolism, and performance of broilers. Animals: Open Access J MDPI. 2021;11(5):1286. [DOI] [PMC free article] [PubMed]
- 17.Bohler MW, Chowdhury VS, Cline MA, Gilbert ER. Heat stress responses in birds: A review of the neural components. Biology. 2021;10(11):1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oladokun S, Adewole DI. Biomarkers of heat stress and mechanism of heat stress response in avian species: current insights and future perspectives from poultry science. J Therm Biol. 2022;110:103332. [DOI] [PubMed] [Google Scholar]
- 19.Kadhim HJ, Kang SW, Kuenzel WJ. Differential and Temporal expression of Corticotropin releasing hormone and its receptors in the nucleus of the hippocampal commissure and paraventricular nucleus during the stress response in chickens (Gallus gallus). Brain Res. 2019;1714:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Wasti S, Sah N, Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10(8):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu AN, Zhang KY, Wang JP, Bai SP, Zeng QF, Peng HW, Ding XM. Effect of different concentrations of neohesperidin dihydrochalcone on performance, egg quality, serum biochemistry and intestinal morphology in laying hens. Poult Sci. 2021;100(7):101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodin L, Sécula A, Chapuis H, Cornuez A, Lessire M, Cobo E, Marie-Louise S, Bonnefont CMD, Barrieu J, Mercerand F, et al. Dietary methionine deficiency reduces laying performances of female common ducks and impacts traits of interest of their mule ducklings. Poult Sci. 2019;98(11):5590–600. [DOI] [PubMed] [Google Scholar]
- 23.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–83. [DOI] [PubMed] [Google Scholar]
- 24.DeGroat W, Mendhe D, Bhusari A, Abdelhalim H, Zeeshan S, Ahmed Z. IntelliGenes: a novel machine learning pipeline for biomarker discovery and predictive analysis using multi-genomic profiles. Bioinformatics. 2023;39(12):btab755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reel PS, Reel S, Pearson E, Trucco E, Jefferson E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol Adv. 2021;49:107739. [DOI] [PubMed] [Google Scholar]
- 26.Gu M, Li C, Chen L, Li S, Xiao N, Zhang D, Zheng X. Insight from untargeted metabolomics: revealing the potential marker compounds changes in refrigerated pork based on random forests machine learning algorithm. Food Chem. 2023;424:136341. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Shi M, Chen L, Zhang S, Chi S, Dong X, Deng J, Tan B, Xie S. Effects of bile acids supplemented into low fishmeal diet on growth, molting, and intestinal health of Pacific white shrimp, Litopenaeus vannamei. Aquaculture Rep. 2023;29:101491. [Google Scholar]
- 28.Shen Y, Wang X, Yuan R, Pan X, Yang X, Cai J, Li Y, Yin A, Xiao Q, Ji Q, et al. Prostaglandin E1 attenuates AngII-induced cardiac hypertrophy via EP3 receptor activation and Netrin-1upregulation. J Mol Cell Cardiol. 2021;159:91–104. [DOI] [PubMed] [Google Scholar]
- 29.Iskusnykh IY, Zakharova AA, Pathak D. Glutathione in Brain Disorders and Aging. Molecules. Molecules (Basel, Switzerland). 2022;27(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berglund T, Wallström A, Nguyen TV, Laurell C, Ohlsson AB. Nicotinamide; antioxidative and DNA hypomethylation effects in plant cells. Plant Physiol Biochem. 2017;118:551–60. [DOI] [PubMed] [Google Scholar]
- 31.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Pan X, Zhao L, Yang C, Zhang Z, Wang B, Gao Z, Jiang K, Ye Y, Wang S, et al. Immune cell infiltration signatures identified molecular subtypes and underlying mechanisms in gastric cancer. NPJ Genomic Med. 2021;6(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson M, Rashidi N, Nurgali K, Apostolopoulos V. The role of Tryptophan metabolites in neuropsychiatric disorders. Int J Mol Sci. 2022;23(17):9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teleanu RI, Niculescu AG, Roza E, Vladâcenco O, Grumezescu AM, Teleanu DM. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int J Mol Sci. 2022;23(11):5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho DM, Artavanis-Tsakonas S, Louvi A. The Notch pathway in CNS homeostasis and neurodegeneration. Wires Dev Biol. 2020;9(1):e358. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Cai H, Han Y, Yang P. Mechanisms of Heat Stress on Neuroendocrine and Organ Damage and Nutritional Measures of Prevention and Treatment in Poultry. Biology (Basel). 2024;13(11):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stojilkovic SS. Signaling pathways regulating pituitary functions. Mol Cell Endocrinol. 2018;463:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman MS, Hossain KS, Das S, Kundu S, Adegoke EO, Rahman MA, Hannan MA, Uddin MJ, Pang MG. Role of Insulin in Health and Disease: An Update. Int J Mol Sci. 2021;22(12):6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dard A, Weiss A, Bariat L, Auverlot J, Fontaine V, Picault N, Pontvianne F, Riondet C, Reichheld JP. Glutathione-mediated thermomorphogenesis and heat stress responses in Arabidopsis thaliana. J Exp Bot. 2023;74(8):2707–25. [DOI] [PubMed] [Google Scholar]
- 40.Boknik P, Eskandar J, Hofmann B, Zimmermann N, Neumann J, Gergs U. Role of Cardiac A2A Receptors Under Normal and Pathophysiological Conditions. Front Pharmacol. 2021;11:627838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Álvarez-Santos MD, Álvarez-González M, Estrada-Soto S, Bazán-Perkins B. Regulation of Myosin Light-Chain Phosphatase Activity to Generate Airway Smooth Muscle Hypercontractility. Front Physiol. 2020;11:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis RL. Adrenergic and noradrenergic regulation of poultry behavior and production. Domest Anim Endocrinol. 2016;56:S94–100. [DOI] [PubMed] [Google Scholar]
- 43.Su M, Paknejad N, Zhu L, Wang J, Do HN, Miao Y, Liu W, Hite RK, Huang X-Y. Structures of β1-adrenergic receptor in complex with Gs and ligands of different efficacies. Nat Commun. 2022;13(1):4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landstrom AP, Adekola BA, Bos JM, Ommen SR, Ackerman MJ. PLN-encoded phospholamban mutation in a large cohort of hypertrophic cardiomyopathy cases: summary of the literature and implications for genetic testing. Am Heart J. 2011;161(1):165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodeify R, Kreydiyyeh S, Zaid LMJ. Identified and potential internalization signals involved in trafficking and regulation of Na+/K + ATPase activity. Mol Cell Biochem. 2024;479(7):1583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Zhao X, Yu M, Zhang M, Feng J. Effects of heat stress on breast muscle metabolomics and lipid metabolism related genes in growing broilers. Animals. 2024;14(3):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simental-Mendía LE, Simental-Mendía M, Sánchez-García A, Banach M, Serban M-C, Cicero AFG, Sahebkar A. Impact of ursodeoxycholic acid on Circulating lipid concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Lipids Health Dis. 2019;18(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marques C, Hadjab F, Porcello A, Lourenço K, Scaletta C, Abdel-Sayed P, Hirt-Burri N, Applegate LA, Laurent A. Mechanistic insights into the multiple functions of niacinamide: therapeutic implications and cosmeceutical applications in functional skincare products. Antioxidants. 2024;13(4):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hii H-P, Lo W-Z, Fu Y-H, Chen M-H, Shih C-C, Tsao C-M, Ka S-M, Chiu Y-L, Wu C-C, Shih C-C. Improvement in heat stress-induced multiple organ dysfunction and intestinal damage through protection of intestinal goblet cells from prostaglandin E1 analogue Misoprostol. Life Sci. 2022;310:121039. [DOI] [PubMed] [Google Scholar]
- 50.Han Z, Xu Z, Xu Y, Lin J, Chen X, Wang Y, Yu Q, Li C, Chen D, Hu H, et al. Phylogenomics reveal DcTPS-mediated terpenoid accumulation and environmental response in Dendrobium catenatum. Ind Crops Prod. 2024;208:117799. [Google Scholar]
- 51.Liu Z, Wang H, Zhang J, Chen Q, He W, Zhang Y, Luo Y, Tang H, Wang Y, Wang X. Comparative metabolomics profiling highlights unique color variation and bitter taste formation of Chinese Cherry fruits. Food Chem. 2024;439:138072. [DOI] [PubMed] [Google Scholar]
- 52.Le PM, Benhaddou-Andaloussi A, Elimadi A, Settaf A, Cherrah Y, Haddad PS. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J Ethnopharmacol. 2004;94(2):251–9. [DOI] [PubMed] [Google Scholar]
- 53.Pritchett EM, Van Goor A, Schneider BK, Young M, Lamont SJ, Schmidt CJ. Chicken pituitary transcriptomic responses to acute heat stress. Mol Biol Rep. 2023;50(6):5233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng S, He YX, Zeng T, Wang DQ, He J, Xia Q, Zhou CY, Pan DD, Cao JX. Heat stress induces various oxidative damages to myofibrillar proteins in ducks. Food Chem. 2022;390:133209. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Shao S, Zeng Y, Wang X, Qin Y, Ren Q, Xiang S, Wang Y, Xiao J, Sun Y. Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nat Cell Biol. 2022;24(3):340–52. [DOI] [PubMed] [Google Scholar]
- 56.Yang S, Zhao T, Ma A, Huang Z, Yang J, Yuan C, Guo X, Zhu C. Heat stress-induced HSP90 expression is dependent on ERK and HSF1 activation in turbot (Scophthalmus maximus) kidney cells. Cell Stress Chaperones. 2021;26(1):173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–115. [DOI] [PubMed] [Google Scholar]
- 58.Silva PS, Hooper HB, Manica E, Merighe GKF, Oliveira SA, Traldi AS, Negrao JA. Heat stress affects the expression of key genes in the placenta, placental characteristics, and efficiency of Saanen goats and the survival and growth of their kids. J Dairy Sci. 2021;104(4):4970–9. [DOI] [PubMed] [Google Scholar]
- 59.Pires BV, Stafuzza NB, de Freitas LA, Mercadante MEZ, Ramos ES, Paz CCP. Expression of candidate genes for residual feed intake in Tropically adapted Bos taurus and Bos indicus bulls under thermoneutral and heat stress environmental conditions. J Therm Biol. 2021;99:102998. [DOI] [PubMed] [Google Scholar]
- 60.Cramer T, Rosenberg T, Kisliouk T, Meiri N. Early-life epigenetic changes along the corticotropin-releasing hormone (CRH) gene influence resilience or vulnerability to heat stress later in life. Mol Psychiatry. 2019;24(7):1013–26. [DOI] [PubMed] [Google Scholar]
- 61.Vom Berg-Maurer CM, Trivedi CA, Bollmann JH, De Marco RJ, Ryu S. The severity of acute stress is represented by increased synchronous activity and recruitment of hypothalamic CRH neurons. J Neurosci. 2016;36(11):3350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao A, Jin X, Pang J, Moskophidis D, Mivechi NF. The transcriptional regulator of the chaperone response HSF1 controls hepatic bioenergetics and protein homeostasis. J Cell Biol. 2017;216(3):723–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Be. 2012;100(4):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munguba H, Srivastava I, Gutzeit VA, Singh A, Vijay A, Kristt M, Arefin A, Thukral S, Broichhagen J, Stujenske JM, et al. Projection-targeted photopharmacology reveals distinct anxiolytic roles for presynaptic mGluR2 in prefrontal- and insula-amygdala synapses. Neuron. 2025;113(6):912-30.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang YY, Cai HY, Han YS, Yang PL. Mechanisms of Heat Stress on Neuroendocrine and Organ Damage and Nutritional Measures of Prevention and Treatment in Poultry. Biology-Basel. 2024;13(11):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kny M, Standera S, Hartmann-Petersen R, Kloetzel PM, Seeger M. Herp regulates Hrd1-mediated ubiquitylation in a ubiquitin-like domain-dependent manner. J Biol Chem. 2011;286(7):5151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shinozaki S, Chiba T, Kokame K, Miyata T, Kaneko E, Shimokado K. A deficiency of herp, an Endoplasmic reticulum stress protein, suppresses atherosclerosis in ApoE knockout mice by attenuating inflammatory responses. PLoS ONE. 2013;8(10):e75249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paredes F, Parra V, Torrealba N, Navarro-Marquez M, Gatica D, Bravo-Sagua R, Troncoso R, Pennanen C, Quiroga C, Chiong M, et al. HERPUD1 protects against oxidative stress-induced apoptosis through downregulation of the inositol 1,4,5-trisphosphate receptor. Free Radical Bio Med. 2016;90:206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho DV, Chan JY. Induction of Herpud1 expression by ER stress is regulated by Nrf1. FEBS Lett. 2015;589(5):615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walz C, Brenmoehl J, Trakooljul N, Noce A, Caffier C, Ohde D, Langhammer M, Wimmers K, Ponsuksili S, Hoeflich A. Control of Protein and Energy Metabolism in the Pituitary Gland in Response to Three-Week Running Training in Adult Male Mice. Cells. 2021;10(4):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X, Ma Z, Wang Y, Jia H, Wang Z, Zhang L. Heat stress exposure cause alterations in intestinal microbiota, transcriptome, and metabolome of broilers. Front Microbiol. 2023;14:1244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yaguchi Y, Tachikawa M, Zhang ZY, Terasaki T. Organic Anion-Transporting polypeptide 1a4 (Oatp1a4/Slco1a4) at the Blood-Arachnoid barrier is the major pathway of Sulforhodamine-101 clearance from cerebrospinal fluid of rats. Mol Pharmaceut. 2019;16(5):2021–7. [DOI] [PubMed] [Google Scholar]
- 73.Dossa AY, Escobar O, Golden J, Frey MR, Ford HR, Gayer CP. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am J Physiol Gastrointest Liver Physiol. 2016;310(2):G81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pi Y, Wu Y, Zhang X, Lu D, Han D, Zhao J, Zheng X, Zhang S, Ye H, Lian S, et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome. 2023;11(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Renga B, D’Amore C, Cipriani S, Mencarelli A, Carino A, Sepe V, Zampella A, Distrutti E, Fiorucci S. FXR mediates a chromatin looping in the GR promoter thus promoting the resolution of colitis in rodents. Pharmacol Res. 2013;77:1–10. [DOI] [PubMed] [Google Scholar]
- 76.Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50(9):1721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, et al. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc Natl Acad Sci U S A. 2003;100(7):4132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H, Yang L. Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J Mol Endocrinol. 2016;57(2):R93–108. [DOI] [PubMed] [Google Scholar]
- 79.Wu F, Zhang C, Zhao C, Wu H, Teng Z, Jiang T, Wang Y. Prostaglandin E1 inhibits GLI2 Amplification-Associated activation of the Hedgehog pathway and drug refractory tumor growth. Cancer Res. 2020;80(13):2818–32. [DOI] [PubMed] [Google Scholar]
- 80.de Guia RM, Hassing AS, Ma T, Plucinska K, Holst B, Gerhart-Hines Z, Emanuelli B, Treebak JT. Ablation of Nampt in AgRP neurons leads to neurodegeneration and impairs fasting- and ghrelin-mediated food intake. FASEB J. 2021;35(5):e21450. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Q, Li Z, Li Q, Trammell SA, Schmidt MS, Pires KM, Cai J, Zhang Y, Kenny H, Boudina S, et al. Control of NAD(+) homeostasis by autophagic flux modulates mitochondrial and cardiac function. EMBO J. 2024;43(3):362–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J Neuroendocrinol. 2012;24(7):1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daghir NJ. Poultry production in hot climates: Cabi; 2008.
- 84.Merighi S, Gessi S, Borea PA. Adenosine Receptors: Structure, Distribution, and Signal Transduction. In: Borea PA, Varani K, Gessi S, Merighi S, Vincenzi F, editors. The Adenosine Receptors. Cham: Springer International Publishing; 2018. p. 33–57. [Google Scholar]
- 85.Hunt LA, Hospers L, Smallcombe JW, Mavros Y, Jay O. Caffeine alters thermoregulatory responses to exercise in the heat only in caffeine-habituated individuals: a double-blind placebo-controlled trial. J Appl Physiol. 2021;131(4):1300–10. [DOI] [PubMed] [Google Scholar]
- 86.Emrick MA, Sadilek M, Konoki K, Catterall WA. β-Adrenergic–regulated phosphorylation of the skeletal muscle CaV1.1 channel in the fight-or-flight response. Proc Natl Acad Sci U S A. 2010;107(43):18712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun X, Wang W, Dong Y, Wang Y, Zhang M, Wang Z, Yu X, Huang J, Cai H. Relationship between calcium circulation-related factors and muscle strength in rat sciatic nerve injury model. Iran J Basic Med Sci. 2020;23(5):654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Y, Li XM, Zhang M-h, Feng J-h. Effect of relative humidity at either acute or chronic moderate temperature on growth performance and droppings’ corticosterone metabolites of broilers. J Integr Agric. 2019;18(1):152–9. [Google Scholar]
- 89.Lin X, Yang F, Zhou L, Yin P, Kong H, Xing W, Lu X, Jia L, Wang Q, Xu G. A support vector machine-recursive feature elimination feature selection method based on artificial contrast variables and mutual information. J Chromatogr B. 2012;910:149–55. [DOI] [PubMed] [Google Scholar]
- 90.Liu P, Luo N, Liu D, Ying F, Zhu D, Wen J, Zhao G, An B. Integrating GWAS and transcriptomics to identify candidate genes conferring relative growth rate trait in white-feathered broiler. Poult Sci. 2024;103(12):104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12(3):R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The metabolites categories
Additional file 2: Figure S1. OPLS-DA permutation test plot
Additional file 3: Table S2. The details of 149 DAMs
Additional file 4: Figure S2. Characteristic distribution of the Top 50 DAMs
Additional file 5: Table S3. The class proportion of DAMs
Additional file 6: Table S4. The enrichment details of DAMs
Additional file 7: Figure S3. Volcano plot of the differentially expression genes in hypothalamus (A) and pituitary (B)
Additional file 8: Table S5. The details of 160 hypothalamic DEGs
Additional file 9: Table S6. The details of 198 pituitary DEGs
Additional file 10: Figure S4. Interaction network of differentially expressed genes
Additional file 11: Table S7. The details of PPI network topology
Additional file 12: Figure S5. Bar plot of GO terms (Top 60) enriched for hypothalamus (A) and pituitary (B) differentially expressed genes
Additional file 13: Table S8. The enrichment details of hypothalamic DEGs
Additional file 14: Table S9. The enrichment details of pituitary DEGs.
Additional file 15: Table S10. The temperature monitoring records of HS
Additional file 16: Table S11. Pre-processing of serum samples before metabolome sequence
Additional file 17: Table S12. The gradient program of ultra-performance liquid chromatography -tandem mass spectrometry system (comprised an ultra-performance liquid chromatography system and a high-resolution mass spectrometer)
Additional file 18: Figure S6. Correlation matrix diagram of quality control samples
Additional file 19: Table S13. RNA-seq Data Production and Quality Assessment Metrics
Data Availability Statement
The RNA-seq data generated in this study have been deposited in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under the accession number SUB14651504 and the BioProject number PRJNA1230596.The metabolomics dataset generated in this study has been submitted to the China National Center for Bioinformation (CNCB) OMIX database under the accession number OMIX009324 and the BioProject number PRJCA036939.