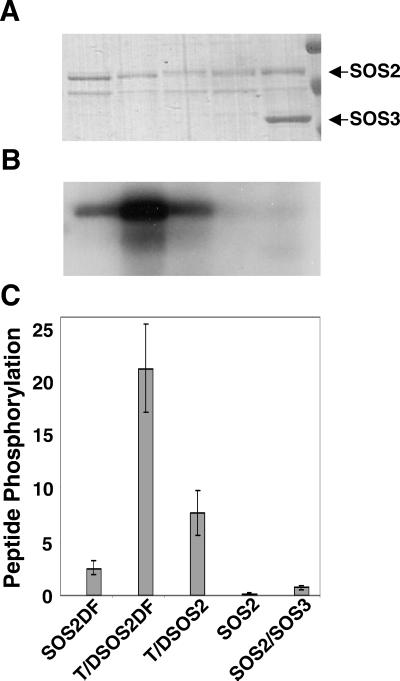
Figure 4.
T/DSOS2DF is a constitutive, highly active form of the SOS2 serine/threonine protein kinase. The kinase activities (autophosphorylation and phosphorylation of an in vitro substrate) of altered forms of the serine/threonine kinase SOS2 (GST-fusion proteins of SOS2DF, T/DSOS2DF, and T/DSOS2) were evaluated. After the autophosphorylation assays, protein (100 ng per lane) was separated by SDS/PAGE, and the gel was stained with Coomassie blue (A) and exposed to x-ray film (B). The arrows indicate SOS2 and SOS3 proteins. (C) The ability of the same GST-SOS2 fusion proteins to phosphorylate the peptide substrate p3 (400 pmol per assay) was determined. The units of protein phosphorylation are nmol/min per mg of protein.