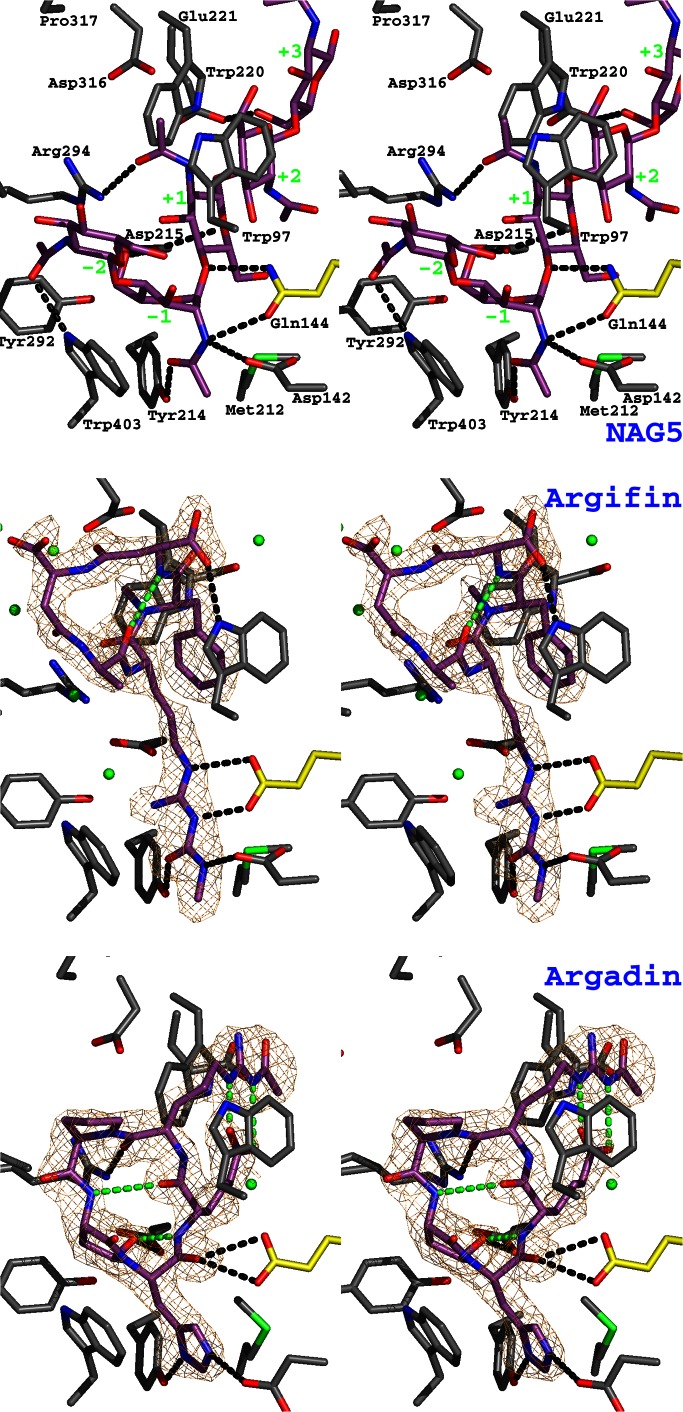
Figure 2.
Argifin and argadin complexed to ChiB. The previously published structure of mutationally inactivated ChiB (where the catalytic Glu-144 has been replaced with a glutamine) in complex with GlcNAc5 (NAG5; ref. 17) is shown as a stereo stick model and compared with the ChiB–argifin and ChiB–argadin complexes. Unbiased (i.e., before including any inhibitor model) |Fo − Fc|,φcalc (contoured at 2.5 σ) maps are shown in orange. Ligand carbon atoms are colored purple. Side chains interacting with the cyclopentapeptides are shown in a sticks representation with carbons colored gray except for the catalytic residue 144, for which carbons are shown in yellow. Tyr-145 (which only hydrogen-bonds to GlcNAc5; see also Fig. 3) has been omitted to improve clarity. Water molecules hydrogen-bonding to both protein and inhibitor are shown as green spheres (hydrogen bonds are not shown). Hydrogen bonds between the ligands and the protein side chains are shown as black dotted lines. Argifin/argadin intramolecular hydrogens bonds are shown as green dotted lines. In the complex with GlcNAc5, the sugar subsites are indicated by green labels.