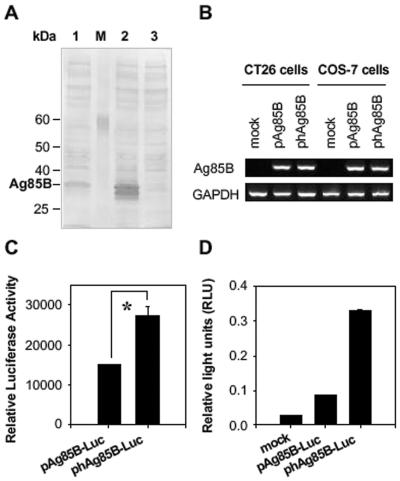
FIG. 2.
Increased expression of Ag85B by codon optimization. (A) Comparative Ag85B expression in COS-7 cells transfected with pAg85B (lane 1) and phAg85B (lane 2) constructs. As a control, COS-7 cells were transfected with mock plasmid (lane 3). Cell lysates (400 μg of total protein) were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and analyzed by immunoblotting with Ag85B-specific polyclonal antibody. The arrow at the right indicates the position of the Ag85B protein. The sizes of protein standards (lane M) are indicated in kilodaltons (kDa) at the left. (B) Total RNA was extracted and analyzed for relative levels of Ag85B mRNA by RT-PCR. Agarose gels were scanned by densitometer to measure the densities of bands. The ratio of Ag85B to GAPDH was calculated and used to obtain the ratio of Ag85B transcripts (Ag85B:hAg85B). Data are representative of at least two independent experiments. (C) Comparison of translational efficacy of pAg85B-Luc and phAg85B-Luc. COS-7 cells were transiently transfected with each plasmid, and 48 h after the transfection, cells were lysed, and luciferase activities were assayed. Data represent relative luciferase activities, with transfection efficiencies normalized by β-galactosidase activity. One representative of at least three performed experiments is shown. *, P < 0.03. (D) For the in vivo expression assay, 200 μg of luciferase-expressing plasmids was intramuscularly injected into the right tibialis anterior muscle of BALB/c mice (2 mice per group), and RLU were determined 14 days later. Data are representative of at least three independent experiments.