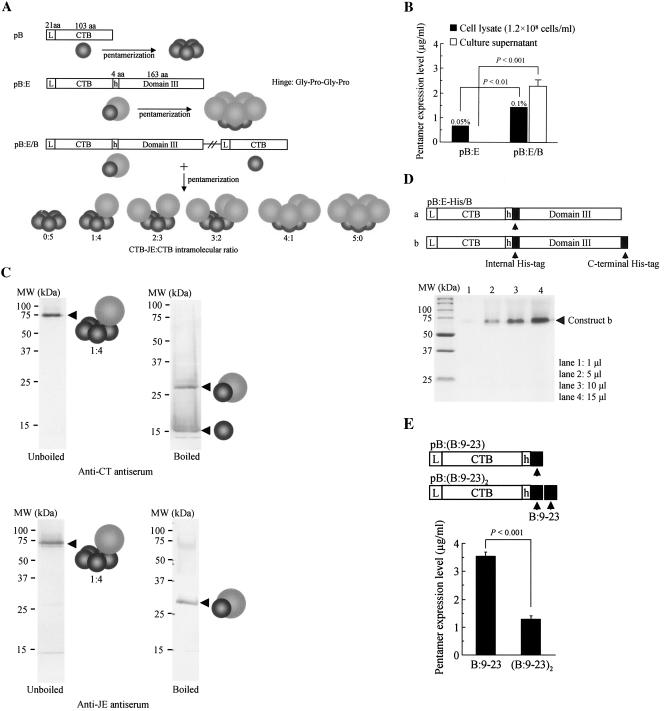
FIG. 2.
Construction and expression analysis of heteropentameric CTB chimeric fusion genes in P. pastoris. (A) A schematic representation of three CTB-related gene constructs and their expected gene products. Two separate CTB fusion expression plasmid vectors were constructed based on the plasmid pBh and pB: pB:E, CTB gene fused in frame with a sequence encoding one-third of the C-terminal region of the JE virus E glycoprotein (domain III; 163 amino acids); pB:E/B, plasmid containing two expression cassettes for CTB fusion and unfused proteins derived from pB:E and pB, respectively. Expected single subunit gene products and their pentamer molecules were schematically drawn for each construct. In theory, coexpression of the fused and the unfused CTB subunits by the plasmid pB:E/B within a single cellular compartment results in the production of six types of pentameric molecular species (i.e., 0:5 to 5:0). (B) Intra- and extracellular CTB chimeric fusion proteins were analyzed by GM1-ELISA as described in the legend of Fig. 1b. Estimated percent levels of intracellularly expressed pentamer proteins in yeast total soluble protein are provided. Data are expressed as the average concentration of pentamers ± standard errors obtained from several clones, with the highest expression levels screened from up to 50 independent transformants. (C) Western blot analysis of the culture supernatant of recombinant yeast transformed with the plasmid pB:E/B. Both boiled (two right panels) and unboiled (two left panels) samples were reacted with anti-CT (two upper panels) or anti-JE antiserum (two lower panels). Schematic drawings for protein bands indicated by arrowheads are the predicted molecular species based on their molecular size and specific reactivity of immune sera. (D) Based on the observation that the heteropentameric construct, unlike homopentamers, was secreted into culture medium, two additional plasmids for expression of heteropentamers with six-His tags were engineered (pB:E-His/B), and secreted proteins purified with the N2+ column were analyzed by SDS-PAGE, followed by Coomassie brilliant blue staining. For conciseness, only the CTB fusion genes of the heteropentameric constructs were drawn. The gel picture shows the purified protein bands of the construct (b) containing two histidine tags (both internal and the C-terminal), with increasing volumes of eluted samples from the N2+ column. (E) Effects on the secretory property of homopentamers by fusion of a short oligopeptide to the CTB molecule were evaluated. A 15-amino-acid T-cell epitope of human insulin B chain (B:9-23) was selected as a model oligopeptide to be fused at the C terminus of the CTB molecule. Two separate expression plasmid vectors were engineered: the CTB gene fused with an oligonucleotide encoding the B:9-23 epitope [pB:(B:9-23)] and the CTB gene fused with two tandemly linked epitope genes [pB:(B:9-23)2]. Secreted expressions were analyzed as described in the legends of Fig. 1b and 2b. Data are expressed as the average concentrations of secreted pentamer proteins ± standard errors from 10 clones, with the highest expression levels screened by GM1-ELISA from up to 50 independent transformants. Statistical significance of the differences in pentamer expression levels was determined by a Mann-Whitney U test. aa, amino acids.