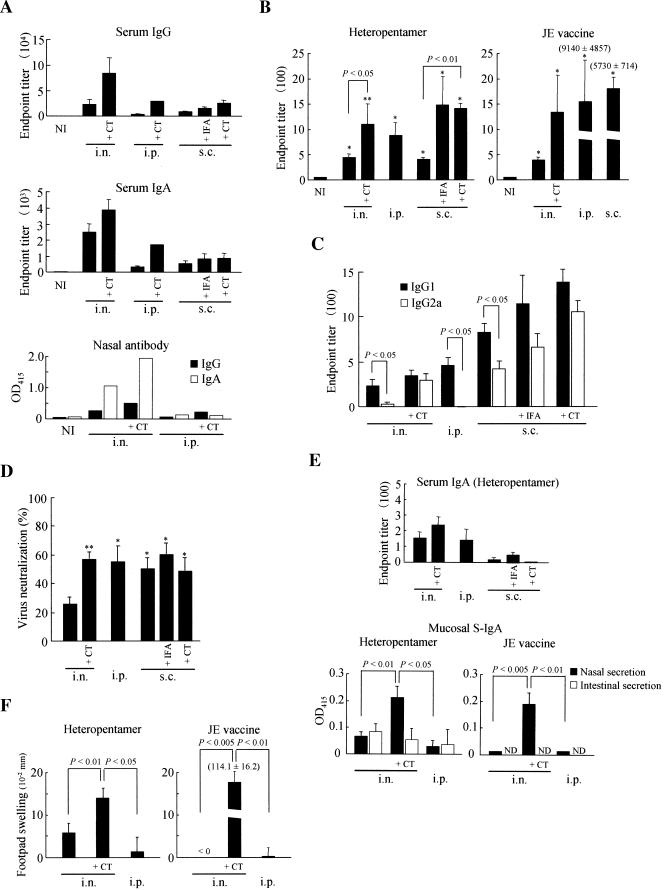
FIG. 3.
Immunogenicity analysis of purified heteropentameric CTB chimeric fusion proteins. (A) Serum and mucosal antibody responses against CTB were examined in BALB/c mice immunized with the heteropentameric protein through the intranasal, intraperitoneal, or subcutaneous route in the presence or absence of indicated adjuvant. Both IgG and IgA isotypes of immunoglobulins were examined from serum and nasal secretions. The data are expressed as endpoint titers ± standard errors for serum antibodies and as the average OD415 for secretory antibodies in the pooled nasal washings. NI, nonimmune samples obtained from unimmunized mice. (B) JE virus-specific serum IgG responses were determined for mice immunized with the heteropentameric protein or the formalin-inactivated JE vaccine. The data are expressed as endpoint titers ± standard errors. NI, nonimmune samples obtained from unimmunized mice. *, P < 0.01 versus NI. **, P < 0.001 versus NI. (C) Virus-specific serum IgG1 and IgG2a subclasses were determined for immune sera from mice immunized with the heteropentameric protein. The data are expressed as endpoint titers ± standard errors as described for panel b. (D) JE virus neutralization antibodies were analyzed in mice immunized with the heteropentameric protein with or without the use of adjuvant as indicated. The data are expressed as percent reductions in PFU ± standard errors for each immune serum diluted 10-fold with PBS. *, P < 0.05; **, P < 0.01 versus intranasal immunization with the heteropentameric protein alone. (E) Virus-specific serum IgA responses were determined for mice immunized with the heteropentameric protein (upper panel). The data are expressed as endpoint titers ± standard errors. Nasal and intestinal washings were analyzed for the presence of virus-specific serum IgA (S-IgA), and the data are expressed as the average OD415 after subtraction of the background levels of samples obtained from naïve mice. Undiluted and fourfold-diluted samples were used in assays for nasal washings of mice immunized with the heteropentameric protein and the JE vaccine, respectively. ND, not determined. (F) DTH responses induced against the formalin-inactivated JE vaccine were determined. The data are expressed as the differences in footpad thickness ± standard errors between the right and the left footpads injected with the JE vaccine and PBS, respectively. Statistical significance of differences was determined by a Mann-Whitney U test. i.n., intranasal; i.p., intraperitoneal; s.c., subcutaneous.