Abstract
In mammals, there exists only scant evidence of female mate choice in species mating on arenas, so-called leks. This has led to hypotheses of lek evolution that are based on benefits to females from reduced harassment by males, low predation risk, or improved availability of scarce nutrients. Here I report that female topi antelopes (Damaliscus lunatus) compete aggressively for matings with preferred males on central lek territories. Females fight at higher rates and more likely disrupt mating attempts of others in the lek center than elsewhere. Contrary to the predictions of the alternative hypotheses, food resources were insignificant, and harassment levels and estimated predation risk were higher on than off lek. These results clearly demonstrate female competition for mates in a lekking mammal in which a female chooses between males for the sole purpose of mating. The finding suggests that the forces leading to lek evolution in mammals and birds may be more similar than previously acknowledged.
Whether female mammals prefer certain males based solely on their role in fertilization is notoriously difficult to demonstrate (1). Species mating on leks offer ideal opportunities for studying female mate choice because males provide no resources except sperm (2, 3). Six of nine species of lekking mammals are ungulates (4). In all lekking ungulate populations, only a fraction of males defend lek territories; others defend more dispersed territories. Several studies have failed to find evidence that females in these species discriminate among mating partners. This failure has led to the suggestion that the skew in male mating success observed in lekking ungulates is merely a byproduct of female movement patterns determined by other factors than mate choice (5–7). Accordingly, ungulate leks are thought to have evolved where females on lek gain direct benefits from avoidance of harassing males (8), low predation risk (5), or the availability of scarce nutrients (9), but evidence to support these hypotheses is wanting (10, 11).
I studied lek-breeding in topi antelopes with the aim of testing the four hypotheses mentioned above. I focused on female behavior and tested the following predictions from those hypotheses: (i) if estrous females benefit from harassment avoidance, harassment levels should be lower on than off lek; (ii) if females gain antipredator benefits, predator density and predator encounter rate should be lower on than off lek; (iii) if females gain nutritional benefits, food availability and feeding rates should be higher on lek than elsewhere; (iv) if females gain benefits from mate choice, they should compete for mating opportunities and have higher probability of mating per unit time on lek than elsewhere.
Methods
Study Area and Animals.
Between February 1998 and June 2000, topi antelopes were studied in the Serengeti–Mara ecosystem. The focal study area contained three leks, each with 11–14 males surrounded by a network of males defending larger territories, that I designate resource territories. More than 90% of the topi calves were conceived during a 1.5 month rut in the long wet season, which typically falls between March and May (12). At this time, most females gathered in the vicinity of the leks. Smaller groups of up to 40 females then moved on to the leks for short periods of time during the day. Females on lek were disproportionately in estrus (≈35%) compared with those off lek (3%) (12). I recognized individuals by using variation in horn morphology, earnicks, coloration, face profile shape and scarring. I assessed the reliability of my identification by using repeated measurements of size as explained below.
Morphological Measurements.
Shoulder height was measured from projected slides of standing individuals calibrated by the projected image of a 1-m pole taken at the same distance; precise distance was measured by using a laser rangefinder (Bushnell Yardage Pro 800). Based on correlation between horn wear and tooth eruption, horn wear was used to estimate age (13) on a sliding scale from 1 to 9. Both male and female topi have horns. Body condition was also scored on a scale from 1 to 9 by using the roundness of the lumbar region and the number of ribs visible (14). Facemasks, which develop shortly after calves turn two months old and do not change outline through life, were scored as dark if males had full black bridges under the eyes and on the muzzle.
Dominance.
Female dominance index was calculated as: (1/N) × Σ(Wi/Ti) where: N = total number of opponents; Wi = number of wins in interactions with opponent i; Ti = total number of interactions with opponent i (15). Females were categorized as relatively subordinate if they scored between 0 and 0.5, and relatively dominant if they scored between 0.5 and 1. I included only unambiguous escalated agonistic encounters when determining the dominance index, i.e., low horn threats, horn clashes, and chases, and these were weighted equally.
Behavioral Observations.
Focal watches were conducted on a total of 55 estrous females, mean watch duration 410 (SE = 31) min. A female was judged to be in estrus if a male sniffed her ano-genital region and became excited. During focal watches, the following information was continuously recorded: identity of the territorial male, the number of other females and predators on the territory, and the activity of the focal female (including agonistic and sexual interactions, chases by males, ground sniffing behavior, and grazing). During a single estrus, which lasts around 24 h, females typically have several mating partners and mate several times with each.
Predation Risk.
I used two measures of predation risk: the mean density of spotted hyenas (Crocuta crocuta) on male territories, and the mean ratio of hyenas to ungulates within a 100-m radius of a female topi during a focal watch. Hyena density was calculated for 64 territories surveyed on average 48 times (range 21–84); territory size was determined in ARCVIEW 3.2 based on repeated locations of territory holders. Hyenas were presumed to be the main predator of topi in the area based on predator counts and prey preference ratings (16–18).
Food Availability.
I measured food availability within each territory by resting a 21 × 21 cm polystyrene tile weighing 25 g on the grass at 25 randomly determined sites. The following measures were taken: greenness (the proportion of the four leaves closest to the corners of the quadrate that was green), grass cover (under the translucent tile), and sward height (from the ground to the center of the tile). Based on the finding that topi select for green leaf in their diet rather than particular grass species (19), I calculated a measure of food availability, the green leaf index, by multiplying the three measures (20).
Statistical Analysis.
To measure female mating preferences the latency to mating was compared across territory types by Kaplan–Meier survival analysis, which determines how long an estrous female “survives” on a territory on average before being mated. For the analysis, territorial visits by focal females in estrus were pooled whether or not mating occurred. Time 0 was defined by the arrival on a territory. As time progressed, visits were excluded either if mating occurred, the female left the territory, or the observation was terminated. The significance of differences between territory types was tested by the Breslow test, which takes into account the decline in sample size over time.
In logistic regression analyses, I modeled (i) whether or not a female mated during a territorial visit, (ii) female dominance status, and (iii) whether or not a female sniffed the ground during a territorial visit. The independent variables were tested by backward regression and only retained in the model if they explained a significant proportion of the variance in the dependent variable.
Other analyses were done by nonparametric statistics. For the analyses of harassment levels, I included only females observed for at least 1 h on all three territory types.
Results and Discussion
Females on leks did not benefit from reduced male harassment as estrous females on lek where chased more frequently (central lek 21.5 per h, peripheral lek 13.0 per h, resource territories 8.9 per h; Friedman: χ2 = 13.27, n = 11 females, P = 0.001) and for longer time (central lek 2.0% of time spent, peripheral lek 1.4%, resource territories 1.0%; Friedman: χ2 = 10.36, n = 11 females, P = 0.006; Dunnett's test: central lek vs. resource territories, P < 0.05 for both chase rate and duration). Antipredator benefits of being on leks were also unlikely because hyena densities were higher on territories closer to the lek center (Spearman: rS = −0.37, n = 64 territories, P = 0.002) and individual females were more likely to encounter hyenas when visiting leks than when visiting dispersed territories (Wilcoxon: Z = −2.34, n = 40 estrous females, P = 0.019). Evidence that visiting leks provided nutritional benefits was negative: the ground on central territories was often worn bare (green leaf index during the rut: central lek territories 24.1, resource territories 100.9; Mann–Whitney: U = 89, n1 = 17, n2 = 27, P = 0.001), and females grazed there only 7% of the time compared with 51% on resource territories (Wilcoxon: Z = −4.05, n = 24 females, P < 0.001).
In contrast, my data support active female mate choice. Females were more likely to mate and to mate sooner after arrival on central lek territories than elsewhere (Table 1; Fig. 1). Several morphological traits of males correlated with their territorial location. The preferred males in the lek center were significantly larger than other territorial males (Mann–Whitney: central vs. peripheral lek males, U = 143.5, n1 = 23, n2 = 26, P = 0.002; central lek males vs. resource territorial males, U = 561.5, n1 = 23, n2 = 72, P = 0.021) and lek males located in more central positions had darker facemasks (Spearman: rS = −0.27, n = 57 lek males, P = 0.042). However, in multivariate analysis the only variable that had a significant effect on whether a female mated independent of territorial centrality was male age as estimated from horn wear (Table 1).
Table 1.
The results of a logistic regression model of whether or not a female mated during a territorial visit
| Parameter | Coefficient | χ2 | df | P |
|---|---|---|---|---|
| Distance to the lek center (log) | −0.96 | 21.88 | 1 | <0.0001 |
| Female dominance | 1.05 | 12.02 | 1 | 0.0005 |
| Presence of other estrous females | 2.05 | 40.27 | 1 | <0.0001 |
| Visit duration | 0.014 | 27.92 | 1 | <0.0001 |
| Male age | 0.17 | 6.13 | 1 | 0.013 |
| Male shoulder height* | 0.087 | 1.43 | 1 | NS |
| Darkness of male facemask | 0.31 | 1.00 | 1 | NS |
| Male ID | – | 22.99 | 61 | NS |
| Female ID | – | 38.72 | 47 | NS |
Based on 393 visits by 48 females to the territories of 62 males. The final model included only significant terms (χ2 = 141.32, df = 5, P < 0.0001); results relating to other variables were obtained by adding them separately to the final model.
, Sample size reduced to 371 because of missing values; the variables in the final model retained their significance in the sample subset. NS, not significant.
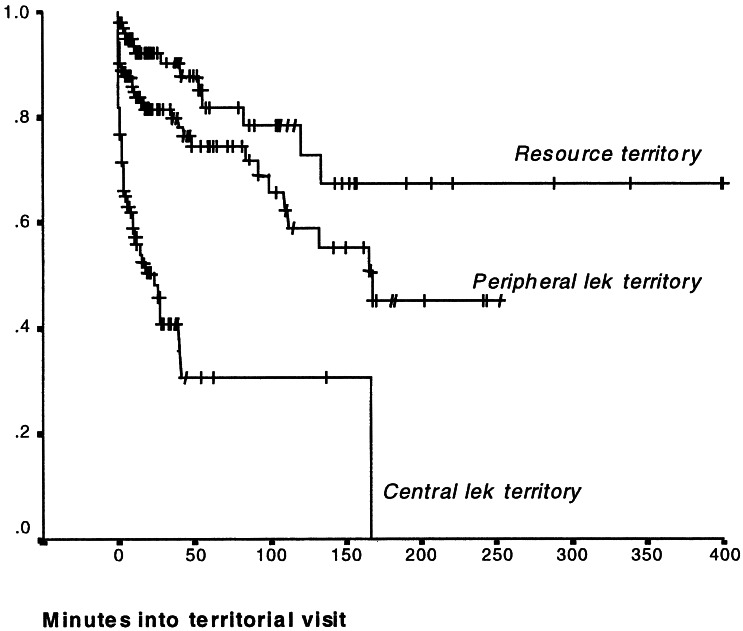
Figure 1.
Kaplan–Meier survival analysis showing the proportion of estrous females that remains unmated in relation to the time since arrival on a territory. Based on 356 territorial visits by 52 estrous females (Breslow test: central vs. peripheral lek territories 27.08, P < 0.0002, central lek vs. resource territories 42.97, P < 0.0001, peripheral lek vs. resource territories 6.66, P = 0.029).
Exceptionally clear evidence of active female mate choice was the fact that females competed aggressively for mating opportunities with preferred males. Escalated agonistic encounters between females were more common in the lek center (Fig. 2a), where females also were more likely to actively disrupt the matings of others (Fig. 2b, Fig. 3). Subordinate females suffered interference from other females during 15% of mating bouts whereas dominant females were disrupted during only 2% of bouts (Mann–Whitney: U = 79, n1 = n2 = 21 females, P < 0.001). This might explain why subordinates were less likely than dominants to mate during a territorial visit (Table 1). Subordinate females were typically young individuals or individuals in poor condition (logistic regression model of dominance: condition × age, χ2 = 6.90, n = 71 females, P = 0.009). Disrupting mating may benefit females because preferred males became visibly exhausted sexually during peaks in mating activity, indicating that their sperm may have become depleted.
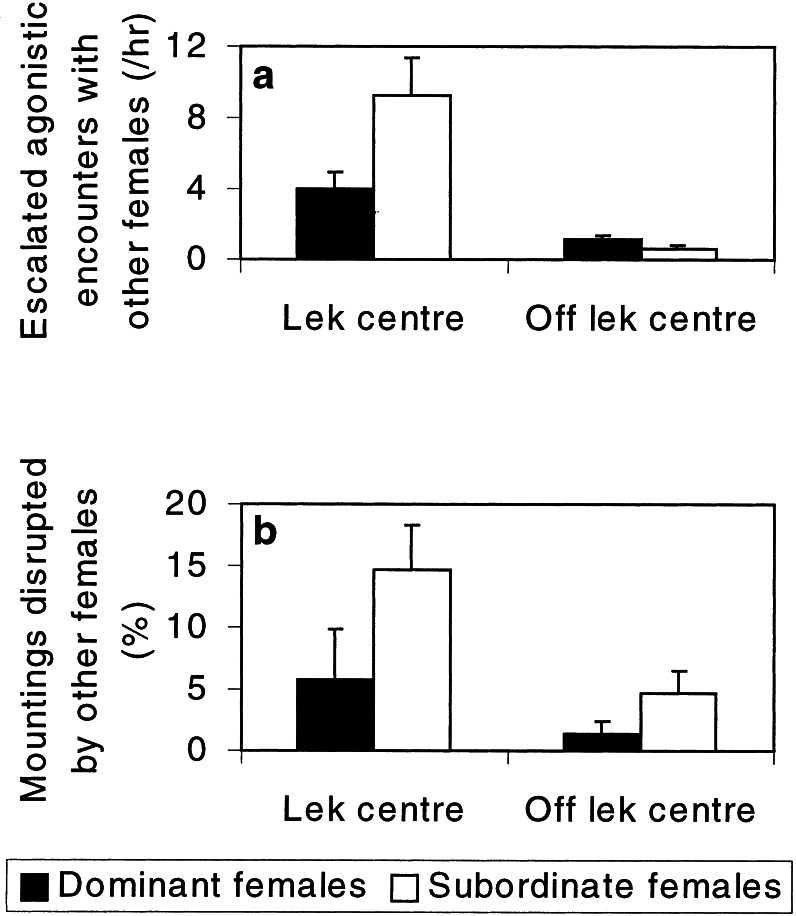
Figure 2.
Female mate competition. (a) The frequency of escalated agonistic encounters experienced by estrous females on and off the lek center (Wilcoxon: subordinate females, Z = −3.41, n = 15, P = 0.001; dominant females, Z = −2.05, n = 15, P = 0.041). (b) The probability of having a mounting disrupted on and off the lek center; only data on focal females seen mating in both locations are included (Wilcoxon, difference between locations: subordinate females, Z = −2.02, n = 6, P = 0.043; dominant females, Z = −0.41, n = 12, not significant). Error bars indicate SE of mean.
Figure 3.
Active mating disruption. (Top) A female repeatedly attacks a central lek male while he mates with another female. (Middle) The male eventually turns around and engages in a fight with the disrupting female. (Bottom) With the disrupted female as a bystander, they drop to their knees as the fighting escalates.
That females mainly based their mate choice on centrality as a way to assess male quality (21) was supported by the overriding influence of territorial location in predicting female mating decisions. Such a female preference could originally have favored high quality males that clustered in areas of higher female densities (22). A positive feedback between genetic benefits of female preference for clustered males and mating benefits to males of clustering would lead to lek formation (23). In support of this model, in all other lekking ungulates, from which data are available, central males are larger (24, 25) and obtain higher mating rates than others (24–27).
Females could use the presence of other estrous females as a cue in mate choice. In topi, females were indeed more likely to mate when other estrous females were present on a territory (Table 1). Furthermore, olfactory cues in the soil could reveal recent presence of estrous females. As reported from other lekking ungulates (28), olfaction played a role in mating activities of topi: females were more likely to sniff the ground on lek territories, especially in the lek center, and ground sniffing was associated with territorial visits during which mating occurred (Table 2). However, as in other ungulates, whether the crucial odor emanates from male or female substances remains unresolved.
Table 2.
The results of a logistic regression model of whether or not a female sniffed the ground during a territorial visit
| Parameter | Coefficient | χ2 | df | P |
|---|---|---|---|---|
| Territory type overall | – | 40.83 | 2 | <0.0001 |
| Central vs. peripheral lek territories | 1.91 | 28.09 | 1 | <0.0001 |
| Peripheral lek vs. resource territories | 2.43 | 8.10 | 1 | 0.0044 |
| Whether mating occurred | 1.85 | 26.10 | 1 | <0.0001 |
| Visit duration | 0.0078 | 5.99 | 1 | 0.014 |
| Male ID | – | 20.96 | 56 | NS |
| Female ID | – | 27.06 | 39 | NS |
Based on 290 visits by 40 estrous females to territories of 57 males. The final model included only significant terms (χ2 = 126.21, df = 4, P < 0.0001); results relating to other variables were obtained by adding them separately to the final model.
The present demonstration of female mate competition in a lekking mammal echoes previous studies on lekking bird species, which have shown aggression between females in relation to mating activities to be widespread among these taxa (29–31). As in ungulates, there is a positive correlation between central position and mating success in lek-breeding birds (32). In black grouse (Tetrao tetrix), females that prefer central males obtain mates with higher lifetime performance (33). These similarities suggest that female preference for centrally located males may have played a major role in the evolution of lek behavior in both birds and ungulates. Indeed, the present findings point to the possibility that female choice is a stronger factor in sexual selection among mammals than widely assumed, but it may be less conspicuous in species where females are not well armed and mating is not spatially clustered on leks.
Acknowledgments
I am grateful to S. Durant, L. M. Gosling, A. Pomiankowski, G. Cowlishaw, M. Petrie, C. Roberts, M. Rowcliffe, H. Kokko, I. P. F. Owens, W. J. Sutherland, T. Dabelsteen, A. F. G. Bourke, and C. B. Müller for valuable advice and other help. For permissions to do field work, I thank the Office of the President and Narok County Council in Kenya, The Commission on Science and Technology and Tanzania Wildlife Research Institute in Tanzania, and the chief park wardens of Masai Mara National Reserve and Serengeti National Park. Funding was provided by the Danish Research Agency, with additional support from the Institute of Zoology at the Zoological Society of London, Ottilie Brorson's Travel Fund, and British Airways.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gibson R M, Langen T A. Trends Ecol Evol. 1996;11:468–470. doi: 10.1016/0169-5347(96)10050-1. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury J W. In: Natural Selection and Social Behaviour. Alexander R D, Tinkle D W, editors. New York: Chiron; 1981. pp. 138–169. [Google Scholar]
- 3.Jennions M D, Petrie M. Biol Rev Cambridge Phil Soc. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- 4.Höglund J, Alatalo R V. Leks. Princeton: Princeton Univ. Press; 1995. [Google Scholar]
- 5.Clutton-Brock T H, Deutsch J C, Nefdt R J C. Anim Behav. 1993;46:1121–1138. [Google Scholar]
- 6.Stillman R A, Clutton-Brock T H, Sutherland W J. Behav Ecol. 1993;4:1–6. [Google Scholar]
- 7.Stillman R A, Deutsch J C, Clutton-Brock T H, Sutherland W J. Anim Behav. 1996;52:891–902. [Google Scholar]
- 8.Clutton-Brock T H, Price O F, MacColl A D C. Behav Ecol. 1992;3:234–242. [Google Scholar]
- 9.McNaughton S J. Nature (London) 1988;334:343–345. doi: 10.1038/334343a0. [DOI] [PubMed] [Google Scholar]
- 10.Carbone C, Taborsky M. Behav Ecol. 1996;7:370–373. [Google Scholar]
- 11.Clutton-Brock T H, McComb K E, Deutsch J C. Behav Ecol. 1996;7:373–378. [Google Scholar]
- 12.Bro-Jørgensen J. Ph.D. thesis. London: Univ. of London; 2001. [Google Scholar]
- 13.Jewell P A. Zool Afr. 1972;7:233–255. [Google Scholar]
- 14.Riney T. J Wildl Mgmt. 1960;24:92–94. [Google Scholar]
- 15.Eden S F. Anim Behav. 1987;35:764–772. [Google Scholar]
- 16.Cooper S M, Holekamp K E, Smale L. Afr J Ecol. 1999;37:149–160. [Google Scholar]
- 17.Ogutu J O, Dublin H T. Afr J Ecol. 1998;36:83–95. [Google Scholar]
- 18.Scheel D. Behav Ecol. 1993;4:90–97. [Google Scholar]
- 19.Duncan P. Ph.D. thesis. Nairobi, Kenya: Nairobi Univ.; 1975. [Google Scholar]
- 20.Balmford A, Rosser A M, Albon S D. Behav Ecol Sociobiol. 1992;31:107–114. [Google Scholar]
- 21.Gosling L M, Petrie M. Anim Behav. 1990;40:272–287. [Google Scholar]
- 22.Sutherland W J, Parker G A. In: Behavioural Ecology—Ecological Consequences of Adaptive Behaviour. Sibly R M, Smith R H, editors. Oxford: Blackwell Scientific; 1985. pp. 255–273. [Google Scholar]
- 23.Sutherland W J. From Individual Behaviour to Population Ecology. Oxford: Oxford University Press; 1996. [Google Scholar]
- 24.Balmford A, Albon S, Blakeman S. Behav Ecol. 1992;3:112–123. [Google Scholar]
- 25.Clutton-Brock T H, Hiraiwa-Hasegawa M, Robertson A. Nature (London) 1989;340:463–465. doi: 10.1038/340463a0. [DOI] [PubMed] [Google Scholar]
- 26.Isvaran K, Jhala Y. Behaviour. 2000;137:547–563. [Google Scholar]
- 27.Nefdt R J C. Ph.D. thesis. Cambridge, U.K.: Cambridge Univ.; 1992. [Google Scholar]
- 28.Deutsch J C, Nefdt R J C. Nature (London) 1992;356:596–598. doi: 10.1038/356596a0. [DOI] [PubMed] [Google Scholar]
- 29.Karvonen E, Rintamäki P T, Alatalo R V. Anim Behav. 2000;59:981–987. doi: 10.1006/anbe.1999.1379. [DOI] [PubMed] [Google Scholar]
- 30.Petrie M, Hall M, Halliday T, Budgey H, Pierpoint C. Behav Ecol Sociobiol. 1992;31:349–358. [Google Scholar]
- 31.Sæther S A, Fiske P, Kålås J A. Proc R Soc London B. 2001;268:2097–2102. doi: 10.1098/rspb.2001.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiske P, Rintamäki P T, Karvonen E. Behav Ecol. 1998;9:328–338. [Google Scholar]
- 33.Kokko H, Rintamäki P, Alatalo R, Höglund J, Karvonen E, Lundberg A. Proc R Soc London B. 1999;266:2109–2115. [Google Scholar]