Abstract
Because of the strain-dependent effect and the lack of simultaneous in vitro test with limited clinical data on Lacticaseibacillus rhamnosus L34 (L34) isolated from the Thai population, L34 was tested and compared with L. rhamnosus GG (LGG). The before and after test using L34 and a randomized placebo-controlled trial using placebo, L34, and LGG, for 4 weeks in patients with non-dialysis chronic kidney disease stage 3–5 (CKD) together with the in vitro experiments using indoxyl sulfate (IS, a representative uremic toxin) were performed. In comparison with the baseline, 4-week-L34 administration reduced gut-derived uremic toxins (GDUTs), except total IS, and attenuated several biomarkers, including i) systemic inflammation, as measured by cytokines and neutrophil extracellular traps using citrullinated histone 3, cell-free DNA, and fluorescent-stained nuclear morphology; ii) gut permeability defect (beta-d-glucan but not by endotoxemia); and iii) gut dysbiosis (fecal microbiome analysis). Additionally, L34-conditioned media attenuated IS-induced injuries on Caco-2 enterocytes, THP-1-derived-macrophages, and isolated neutrophils. Despite the possible different active compounds, both probiotics similarly attenuated IS-induced inflammation in vitro and in patients when compared with the placebo. In conclusion, L34 and LGG similarly attenuated systemic inflammation in patients with CKD, through the improved gut dysbiosis and anti-inflammation.
Keywords: Lacticaseibacillus rhamnosus, Probiotics, Gut-derived uremic toxins, Gut leakage, Chronic kidney disease
Subject terms: Inflammation, Innate immune cells, Chronic kidney disease
Introduction
Chronic kidney disease (CKD), the persistent renal injury at least 3 months, is a rising global healthcare problem with an accumulation of several metabolic substances such as uremic toxins, which mostly derived from metabolic processes1. Due to the declining kidney function, accumulated uremic toxins were alternatively excreted through the intestines, resulting in an imbalance between beneficial and pathogenic bacteria in the gut that is referred to as gut dysbiosis2. In contrast, the dysbiosis triggers production of inflammatory mediators and facilitates translocation of inflammatory molecules into the systemic circulation3–5. Most of the toxins are derived from regular metabolisms, whereas some uremic toxins are synthesized from the dietary compounds, which is well-known as gut-derived uremic toxins (GDUTs), such as indoxyl sulfate (IS) and p-cresol2, that are normally excreted through the urine6. In term of renal injury, uremic toxins always accumulate in the blood, distribute throughout the body, worsen organ damage (renal cell, intestinal epithelium, and vascular endothelium), and activate systemic inflammation7,8. With gut dysbiosis, the production of GDUTs is more prominent than the normal gut eubiosis3, which causes more severe intestinal barrier impairment, inducing the translocation of lipopolysaccharide (LPS) and (1→3)-β-d-glucan (BG), two of which are the major component of Gram-negative bacteria and fungi, respectively, from the gut into the blood circulation and exacerbating CKD progression2,5,9. The correlation among gut dysbiosis, leaky gut, and GDUT-induced CKD progression, called as gut-kidney axis, is a problematic vicious cycle10,11 and the breakdown of the cycle is beneficial4,12,13. Because uremia induces gut dysbiosis and the dysbiosis enhances GDUTs, the treatment of gut dysbiosis14,15 that might reduce GDUTs and cytokines16,17 is one of the interesting strategies to break the vicious cycle of gut-kidney axis.
Previous studies revealed that the administration of several probiotics (bacteria with health benefits) can mitigate CKD-induced gut dysbiosis and systemic inflammation in animal models and in patients18,19. Therefore, probiotics might be beneficial in CKD with gut permeability defect (leaky gut). Almost all of the patients with pre-dialysis CKD might already have leaky gut20, which is not only associated with CKD severity but might be correlated with several environmental factors, including drugs, diets, and underlying diseases, that might be different in various regions of the world. For example, spicier foods with more stressful social environments might induce more severe gut dysbiosis21,22 in some countries than others. Hence, the effect of probiotics might be different in the dissimilar environment, partly due to the culture variety, and the probiotics isolated from the specific population might also be different because of the microbiological environment. In addition, the tolerance against capsaicin (an active molecule from chili) of Lacticaseibacillus rhamnosus L34 (L34), which was isolated from the Thai population (people with regular spicy diets), is more prominent than Lacticaseibacillus rhamnosus GG (LGG), which was isolated from others23. Notably, L34 is originally isolated from Thai newborn feces, which possibly pass through from mothers24; however, L34 and Lacticaseibacillus spp. are rarely found in adult Thai volunteers according to our previous publication23, implying a requirement for the oral administration of the probiotics. As such, the anti-inflammatory properties of L34 are mentioned in the conditions with uremia-induced gut permeability defect and other animal models with limited use in patients with CKD. Indeed, uremia causes gut leakage, enhances systemic inflammation, facilitates CKD progression, and worsens renal fibrosis25, that might be neutralized by probiotics with anti-inflammatory properties26–28. Here, we hypothesized that L34, which is a local probiotic strain, might be useful in CKD similar to LGG, which is a standard strain with substantial supported evidence. Then, the before and after experiments using L34 was conducted as a proof of concept before performing a randomized control trial using placebo, L34 and LGG, together with the in vitro experiments on Caco-2 enterocytes, THP-1-derived macrophages, and isolated neutrophils from healthy volunteers.
Methods
Enrolled participants and sample analysis
The study protocol was approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University (IRB number 0055/66) with the informed consents was obtained from all subjects following the STROBE guideline and was adhered to the Declaration of Helsinki. The study was registered to the Thai Clinical Trial Registry on 07/08/2021 (TCTR20210807005). The participants were enrolled for 2 sets of the experiments, including the before and after experiment (pre-post study) and a randomized control trial study, from December 2023 to December 2024. Adults aged ≥ 18 years with non-dialysis CKD stage 3–5 (eGFR 10–60 mL/min/1.73 m2) and received treatment at the King Chulalongkorn Memorial Hospital were recruited. The exclusion criteria containing kidney replacement therapy, the use of products that interfere gut bacteria within 3 months (probiotics, pre-biotics, symbiotics, immunosuppressive agents, and antimicrobial drugs), pregnancy, active bowel inflammation or infection, severe malnutrition (the Subjective Global Assessment class C), active malignancy, or terminal illness. All participants underwent a 4-week run-in period to adjust medications and diets with respect to the KDIGO recommendations for CKD29 with an avoidance for yogurt or fermented foods. Then, the participants were randomly recruited for the pre-post experiments or a randomized control trial. The pre-post experiment was conducted to explore an impact of Lacticaseibacillus rhamnosus L34 (L34), the probiotics extracted from the Thai population, was produced by the Greater Pharma co. (Bang Phlat, Bangkok, Thailand)23. All participants orally received a 3.5 × 109 CFU of L34 coating powder per day in the morning at the same time for 4 weeks with the product count procedure (the participants who missed > 20% of the prescribed doses were excluded from the analysis). Because the once daily administration of L34 at 1 × 109 CFU in healthy volunteers for 20 days successfully sustains the elevation of fecal Lactobacilli in our previous publication23 and the administration of L34 for 1–7 days attenuated inflammation in several models26,27,30–32, the beneficial impacts of L34 might be rapid, especially using the administration for more than 20 days with the increased doses of L34 into 3.5 × 109 CFU. Blood and fecal samples were collected at 1 week before and at 4 weeks after L34 administration (1 day after the administration of L34 in the morning). In parallel, a 4-week administration analysis was conducted to compare the impact of L34 and Lacticaseibacillus rhamnosus GG (LGG) (Chr. Hansen, Hoersholm, Denmark) (3.5 × 109 CFU of LGG coating powder per day in the morning) in comparison with the placebo control (the packages were identical appearance). At 4 weeks after administration, blood sample were collected in all participant groups (placebo, L34, and LGG).
Sample analysis and fecal microbiome
The complete blood count (CBC), blood urea nitrogen (BUN), creatinine (Cr), urine protein creatinine index (UPCI), and serum lactate were measured in the central laboratory of the King Chulalongkorn Memorial Hospital (KCMH) using Sysmex XN9203 Analyzer (Kobe, Hyogo, Japan) and Cobas c502 (Roche Diagnostics, Basel, Switzerland). Serum cytokines (IFN-α, TNF-α, and IL-6) and endotoxin were measured by ELISA (Invitrogen, Waltham, MA, USA) and the Pierce Chromogenic Endotoxin Quant Kit with Limulus Amebocyte Lysate (LAL)-based assay (Thermo Fisher Scientific, Waltham, MA, USA), respectively. The accuracy of endotoxin test is 0.015 to 1.0 EU/mL with the sensitivity at the level less than 0.005 EU/mL. Serum beta-d-glucan was measured by the beta-d-glucan ELISA kit (Assay Genie, Ireland) with the range from 15.6 to 1,000 pg/mL and the sensitivity at 9.4 pg/mL. Meanwhile, total indoxyl sulfate (IS), free IS, and total p-cresol sulfate (p-cresol) were measured by the high-performance liquid chromatography (HPLC Alliance 2695, Waters, Zellik, Belgium) as previously described11,33. Fecal samples were extracted for the metagenomic DNA by FastPrep-24™ Classic bead beating grinder and lysis system (MP Biomedicals, USA) using SPINeasy™ DNA Pro Kit for Feces (MP Biomedicals, USA). The quality of the extracted DNA was determined via DeNovix Fluorometer. The prokaryotic 16S rRNA gene was performed using the Native barcoding. The targeted full length 16S rRNA polymerase chain reaction (PCR) was followed cycling conditions: 95 °C for 1 min, and 40 cycles of 95 °C for 20 s, 55 °C for 30 s and 65 °C for 2 min, followed by 65 °C for 5 min. The 16S rRNA amplicons were purified by the AMPure XP magnetic beads and ligated with different sequencing of Native barcode. The DNA libraries with different barcodes (approximately 1,500 bp) were purified using the AMPure XP beads. The quality and quantity of DNA libraries were evaluated using the DeNovix QFX Fluorometer and the QIAxcel Advanced (Qiagen, Hilden, Germany), respectively. Sequencing was performed using the Oxford nanopore MinION Mk1C platform following the manufacturer’s protocols (Oxford Nanopore Technologies). The base-calling and adapter trimming of raw sequences were performed using the Dorado program version 0.7.3. The sequencing reads were filtered with DADA2 pipeline (minimal length at 1,000 bp and maximal length at 1,600 bp). The high-quality reads were processed following Emu algorithm with Emu database. Emu established microbial community profiles. Alpha diversity index (Chao1 richness, and Shannon) and beta diversity were computed using R software with the pairwise comparison using Kruskal–Wallis test (p < 0.05). Permutational multivariate analysis of variance (PERMANOVA) was performed to evaluate the significant differences for beta diversity among groups at p < 0.05. The Kruskal–Wallis sum-rank test was also used for the Linear discriminant analysis Effect Size (LEfSe) analysis to identify bacterial biomarkers that differed significantly in abundant taxon between sample groups. The nucleic acid sequences were submitted in an open access Sequence Read Archive database of NCBI under the accession number PRJNA1155599. In parallel, the quantification of total fungi was measured by real-time polymerase chain reaction (PCR) which was performed in a QuantStudio™ Design & Analysis Software v1.4.3 and presented the abundance of fecal fungi by the cycle threshold (Ct value) using the Internal Transcribed Spacer (ITS) primer (forward; 5’-TCCGTAGGTGAACCTGCGG-3’ and reverse; 5’-TCCTCCGCTTATTGATATGC-3’) as previously described34. Additionally, citrullinated histone 3 (CitH3) and cell-free DNA in serum were measured by ELISA assay (Cayman Chemical, Ann Arbor, MI, USA) and Quant-iT™ PicoGreen reagents (Thermo Fisher Scientific, Waltham, MA, USA), respectively, according to the manufacturer’s directions35.
Additionally, neutrophil extracellular traps (NETs) were also measured by the staining of nuclear morphology using a fluorescent color; 4′,6-diamidino-2-phenylindole (DAPI) as previously described36,37. Briefly, neutrophils were isolated from blood samples by density centrifugation with the Polymorphprep™ (Axis-Shield, Oslo, Norway) and were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum before placing neutrophils (5 × 105 cells) onto the poly-l-lysine-coated coverslips (6 mm). Then, the coverslips were fixed with 4% formaldehyde, permeabilized with 0.05% Tween-20, blocked with 2% bovine serum albumin (BSA) in 1X Tris-buffered saline (TBS), and stained by DAPI, respectively.
The in vitro experiments of L. rhamnosus conditioned media
To obtain a set of possible direct effects of probiotics on enterocytes, macrophages and neutrophils, IS was used as a representative gut-derived uremic toxin in vitro. The enterocyte cell line (Caco-2 cells; ATCC HTB-37, American Type Culture Collection, Manassas, VA, USA), monocyte-derived macrophages using THP-1 (ATCC TIB-202), and neutrophils from the healthy volunteers were used. Caco-2 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (Invitrogen), while THP-1 were cultured in modified RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), and 1% PenStrep (Gibco), as well as 100 ng/mL of phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) for macrophage differentiation. In parallel, neutrophils were isolated by a gradient separation protocol (density centrifugation) as mentioned above35. Meanwhile, the Lacticaseibacillus conditioned media (LCM) was prepared as a representative of the secreted molecules from the probiotics according to a previous publication26. Briefly, the probiotics at an optical density 600 (OD600) of 0.1 were incubated anaerobically in Lactobacillus MRS broth (Oxoid) for 48 h before collection of the cell-free supernatants by centrifugation and were concentrated by speed vacuum drying at 40°C for 3 h (Savant Instruments). The cell-free concentrated pellets were resuspended in an equal volume of DMEM, stored at −20 °C until use.
Subsequently, at 1 × 106 cells/well of CaCo-2, or THP-1 derived macrophages (THP-1), or neutrophils were incubated with the final concentration of 1 mM of IS (Sigma-Aldrich), according to previous publications7,8, with or without 5% v/v of LCM under 5% CO2 at 37 °C for 24 h. After that, the supernatant was determined for cytokine (IL-8; Invitrogen), whereas cells were used for evaluation the expression of several genes by reverse transcription-polymerase chain reaction (qRT-PCR) with several primers (Table 1)38. The integrity of intestinal cells was determined by Transepithelial electrical resistance (TEER)39, using the monolayer of Caco-2 at 5 × 104 cells per well on the upper compartment of 24-well Boyden chamber trans-well in modified DMEM for 15 days before activation by IS with and without LCM. Then, TEER, in ohm (Ω) × cm2, was measured with epithelial volt-ohm meter (EVOM2™, World precision instruments, Sarasota, FL, USA) by placing electrodes in supernatant at the basolateral chamber and in the apical chamber. The TEER values in media culture without Caco-2 cells was used as a blank and was subtracted from the measured values. For neutrophil extracellular traps (NETs), the CitH3 was measured by Citrullinated Histone H3 ELISA kit (clone 11D3; Cayman Chemical, Ann Arbor, MI, USA) and cell-free DNA in supernatant was measured by Quant-iT™ PicoGreen reagents (Thermo Fisher Scientific, Waltham, MA, USA), while DAPI was stained for the nuclear morphology. To explore the impacts of IS with the more physiologic level, IS at 0.026 mM in 4% heat-inactivated human albumin (Sigma-Aldrich) were used for the determination of some parameters. Notably, the supraphysiologic concentration of IS (1 mM) was used in most of the experiments due to the more prominent cell activation than the IS concentration in the uremic range of patients (0.026 mM). Also, the attenuation effect of LCM against the higher IS concentration more dominantly indicates the beneficial effect of the probiotics than using IS in the lower concentration.
Table 1.
Primer sequences used for qRT-PCR.
| Name | Forward primer | Reverse primer |
|---|---|---|
|
Interleukin-8 (CXCL8) (humans) |
5’- TGGCTCTCTTGGCAGCCTTC −3’ | 5’- TGCACCCAGTTTTCCTTGGG −3’ |
|
Nuclear Factor kappa B (NF-κB) (humans) |
5’- CTTCCTCAGCCATGGTACCTCT −3’ | 5’- CAAGTCTTCATCAGCATCAAACTG −3’ |
| (NOS-2) (humans) | 5’-CAGCGGGATGACTTTCCAAG-3’ | 5’-AGGCAAGATTTGGACCTGCA-3’ |
|
Tight junction Molecule (OCLN) (humans) |
5’-CCAATGTCGAGGAGTGGG-3’ | 5’-CGCTGCTGTAACGAGGCT-3’ |
|
Mucin-2 (MUC-2) (humans) |
5’-CCTGCCGACACCTGCTGCAA-3’ | 5’-ACACCAGTAGAAGGGACAGCACCT-3’ |
| β-actin | 5’-CCTGGCACCCAGCACAAT-3’ | 5’-GCCGATCCACACGGAGTACT-3’ |
Moreover, a preliminary protocol to identify the active substances secreted from LCM (from L34 or LGG) was performed by the neutralization of LCM anti-inflammatory properties (an attenuation in IS-induced inflammation) after processing by enzyme inactivation protocol, using α-amylase, lipase, lysozyme, and proteinase K (Sigma-Aldrich)26. As such, each of the enzyme was incubated in LCM (1 mg/mL) for 6 h at 37 °C for lipase and proteinase K, while using 25°C for amylase and lysozyme. Then, the treated-LCM was tested for inflammatory suppressive activity through the detection of IL-8 and IL-6 for Caco-2 and THP-1-derived macrophages, respectively, and by CitH3 for neutrophils, using different ELISA assays as mentioned above.
Statistical analysis
Analyzed data are presented as mean ± standard error (SE) using GraphPad Prism version 10.4.1 software (La Jolla, CA, USA). Statistical significance was determined by the one-way analysis of variance (ANOVA) followed by Tukey’s analysis for the comparisons more than 2 groups. The paired Student’s t-test was used for the before and after comparison. The p-value of < 0.05 was considered as statistically significant.
Results
L. rhamnosus L34 reduced gut-derived uremic toxins and altered gut microbiota, but not improved kidney function
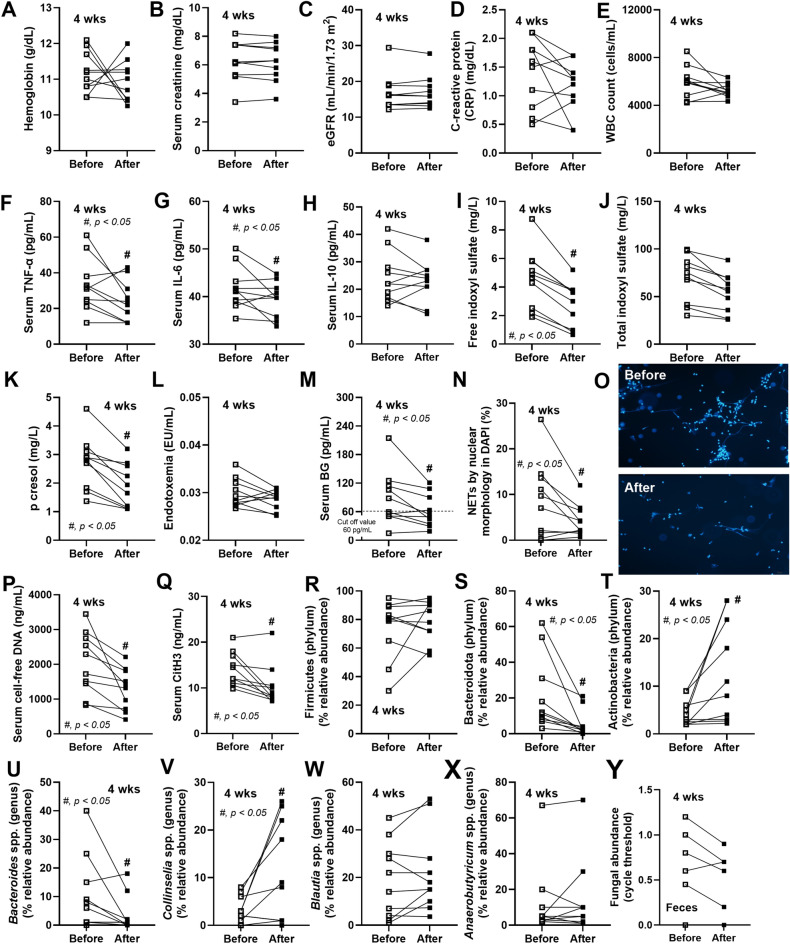
Because the limited data on the use of L34 in patients were differ from LGG, L34 was tested in 10 patients with CKD using a pilot study with a pre-post experiment. The epidemiologic data of these patients was demonstrated in Table 2 and 3. At 4 weeks of probiotic administration, there was no difference in hemoglobin, renal functions as determined by serum creatinine and estimated glomerular filtration rate (eGFR), C-reactive protein (CRP), and total while blood cell (WBC) count, with reduced inflammatory cytokines (TNF-α and IL-6), but not anti-inflammatory cytokine (IL-10), and decreased gut-derived uremic toxins (GDUTs), including p-cresol and free indoxyl sulfate (IS), but not total IS (p = 0.16) (Fig. 1A-K). In parallel, systemic inflammation in patients with CKD was possibly exacerbated by gut permeability defect that was attenuated by L34, as indicated by the reduced serum beta-d-glucan (BG), a major cell wall component of gut fungi, and a trend to decrease in endotoxin (p = 0.23), a major cell wall component of Gram-negative bacteria (Fig. 1L, M). Additionally, L34 attenuated CKD-induced neutrophil extracellular traps (NETs)40, which were determined by nuclear morphology, serum cell-free DNA, and serum citrullinated histone 3 (CitH3) (Fig. 1N-Q). Because probiotics might work in the intestine, fecal microbiome analysis was performed. The alteration after probiotic administration of gut bacterial abundance was mainly in 3 phyla of bacteria, including i) reduced Bacteroidota (the most abundant Gram-negative anaerobes with potential pathogenicity)27,41, ii) increased Actinobacteria (the short chain fatty acid-producing Gram-positive bacteria that might improve gut homeostasis)42, and iii) invariant Firmicutes (the highest Gram-positive anaerobes in healthy gut)43,44 (Fig. 1R-T). Most of bacteria in the genus level of microbiota was not different between pre- and post-probiotic administration (similar Blautia spp. and Anaerobutyricum spp. in phylum Firmicutes), with only a few differences (decreased Bacteroides spp. from phylum Bacteroidota and increased Collinselia spp. from phylum Actinobacteria) (Fig. 1U-X). Meanwhile, there was no difference in fungal abundance in feces with probiotic treatment (Fig. 1Y). The details of fecal microbiome analysis in all bacteriological levels were demonstrated in Fig. 2, while Cladogram (a diagram used to show relations among organisms) was demonstrated in Fig. 3A. With Linear discriminant analysis Effect Size (LEfSe), several Bacteroides spp. were the representatives of the pre-probiotic condition, while L. rhamnosus was the only representative for the post-probiotic condition (Fig. 3B). The principal coordinate analysis (PCoA) on Bray–Curtis (the visualization indicating the differences between microbial communities by projecting data onto a 2-dimensional plot) did not show the categorization of bacteria between the before and after probiotic administration (Fig. 3C). Hence, L34 demonstrated that some beneficial effects in patients with CKD, including the attenuation in GDUTs, leaky gut, and systemic inflammation (cytokines and NETs) with fecal microbiota alteration (Bacteroidota and Actinobacteria) (Fig. 1A-Y). Due to the possible impact of diabetes mellitus (DM) on gut microbiome analysis45, the fecal abundance of patients with DM (IMH004, 005, 008, 010, 034, and 044) and without DM (IMH012, 013, 014, and 024) was demonstrated (Supplementary Fig. 1). There was no significant difference in fecal microbiota between patients with DM and without DM, perhaps due to the limited number of samples, and the tendency of the beneficial impacts of probiotics was similarly demonstrated (Supplementary Fig. 1A-G) compared with the data without the categorization by DM (Fig. 1R-X).
Table 2.
The demographic data of the before and after experiments using L. rhamnosus L 34 (L34).
| Data (mean ± SE*) | n = 10 |
|---|---|
| Age (year) | 53 ± 4 |
| Body weight (kg) | 70 ± 6 |
| Body mass index (BMI) (kg/m2) | 27 ± 3 |
| Hematocrit (%) | 33 ± 4 |
| Hemoglobin (g/dL) | 11 ± 1 |
| Serum sodium (mg/dL) | 138 ± 4 |
| Serum potassium (mg/dL) | 4 ± 1 |
| Serum chloride (mg/dL) | 100 ± 3 |
| Serum bicarbonate (mg/dL) | 23 ± 5 |
| Pitting edema (%) | 2 (20) |
| Serum albumin (mg/dL) | 3.8 ± 0.4 |
| Female (%) | 6 (60) |
| Etiology of CKD**, n (%) | |
| Diabetic nephropathy | 6 (60) |
| Chronic glomerulonephritis | 2 (20) |
| Chronic tubulointerstitial nephritis | 2 (20) |
| Comorbidities, n (%) | |
| Diabetes | 6 (48) |
| Hypertension | 8 (80) |
| Hyperlipidemia | 6 (60) |
| Coronary artery disease | 3 (30) |
| Cerebrovascular disease | 3 (30) |
| Medication, n (%) | |
| ACEI/ARB*** | 10 (100) |
| Diuretics | 3 (30) |
| SGLT2**** inhibitors | 1 (10) |
| Calcium channel blockers | 4 (40) |
| Beta blockers | 8 (80) |
| Statins | 21 (84) |
*, standard error; **, Chronic kidney disease; ***, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; ****, sodium-glucose cotransporter-2.
Table 3.
The demographic data of the cross-sectional experiments using placebo, L. rhamnosus L34 (L34), and L. rhamnosus GG (LGG).
| Data (mean ± SE*) | Placebo (n = 25) | L34 (n = 25) | LGG (n = 25) |
|---|---|---|---|
| Age (year) | 67 ± 6 | 71 ± 8 | 68 ± 12 |
| Body weight (kg) | 73 ± 10 | 75 ± 12 | 71 ± 14 |
| Body mass index (BMI) (kg/m2) | 26 ± 8 | 23 ± 5 | 25 ± 4 |
| Hematocrit (%) | 31 ± 6 | 30 ± 7 | 31 ± 4 |
| Hemoglobin (g/dL) | 10 ± 2 | 11 ± 4 | 11 ± 2 |
| Serum sodium (mg/dL) | 135 ± 5 | 132 ± 6 | 136 ± 4 |
| Serum potassium (mg/dL) | 5 ± 1 | 4 ± 2 | 4 ± 3 |
| Serum chloride (mg/dL) | 98 ± 5 | 100 ± 4 | 97 ± 8 |
| Serum bicarbonate (mg/dL) | 24 ± 4 | 26 ± 2 | 25 ± 2 |
| Pitting edema (%) | 0 (0) | 4 (16) | 3 (12) |
| Serum albumin (mg/dL) | 4.1 ± 0.5 | 4.0 ± 0.3 | 3.9 ± 0.4 |
| Female (%) | 14 (56) | 16 (64) | 19 (76) |
| Etiology of CKD**, n (%) | |||
| Diabetic nephropathy | 12 (48) | 16 (64) | 14 (56) |
| Chronic glomerulonephritis | 8 (32) | 5 (20) | 5 (20) |
| Chronic tubulointerstitial nephritis | 2 (8) | 3 (12) | 3 (12) |
| Others | 3 (12) | 1 (4) | 3 (12) |
| Comorbidities, n (%) | |||
| Diabetes | 12 (48) | 16 (64) | 19 (76) |
| Hypertension | 19 (76) | 20 (80) | 21 (84) |
| Hyperlipidemia | 16 (64) | 17 (68) | 20 (80) |
| Coronary artery disease | 7 (28) | 9 (36) | 6 (24) |
| Cerebrovascular disease | 3 (12) | 4 (16) | 2 (8) |
| Medication, n (%) | |||
| ACEI/ARB*** | 20 (80) | 18 72) | 17 (68) |
| Diuretics | 8 (32) | 7 (28) | 9 (36) |
| SGLT2**** inhibitors | 3 (12) | 2 (8) | 4 (16) |
| Calcium channel blockers | 18 (72) | 17 (68) | 15 (60) |
| Beta blockers | 18 (72) | 20 (80) | 17 (68) |
| Statins | 21 (84) | 20 (80) | 19 (76) |
*, standard error; **, Chronic kidney disease; ***, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; ****, sodium-glucose cotransporter-2.
Fig. 1.
The characteristics of patients with chronic kidney disease (CKD) at before and after 4 week of L. rhamnosus L34 (L34) administration, as indicated by hemoglobin (A), serum creatinine (B), estimated glomerular filtration rate (eGFR) (C), C-reactive protein (CRP) (D), white blood cell count (WBC) (E), serum cytokines (TNF-α, IL-6, and IL-10) (F–H), gut-derived uremic toxins (free indoxyl sulfate, total indoxyl sulfate, and p-cresol) (I-K), endotoxemia (L), serum (1→3)-beta-d-glucan (BG) (M), neutrophil extracellular traps (NETs), as determined by nuclear morphology with representative fluorescent pictures (N, O), cell-free DNA (P), citrullinated histone 3 (CitH3) (Q), the selected graph presentation of fecal bacterial abundance (phylum and genus levels) from fecal microbiome analysis (R-X), and fecal fungal abundance (expressing in cycle threshold or Ct) (Y), are demonstrated (n = 10/group). *, p < 0.05.
Fig. 2.
The fecal microbiome analysis of patients with chronic kidney disease (CKD) at before and after 4 weeks of L. rhamnosus L34 (L34) administration as indicated by the abundance of bacteria in phylum, class, order, family, genus, and species is demonstrated (n = 10/group).
Fig. 3.
The fecal microbiome analysis of patients with chronic kidney disease (CKD) at before and after 4 week of L. rhamnosus L34 (L34) administration as indicated by the Cladogram (a branching diagram showing the cladistic relationship among species) (A), the Linear discriminant analysis Effect Size (LEfSe) (the identified bacteria characterizing the differences between groups) (B), and the principal coordinate analysis (PCoA) (the visualization indicating the differences between microbial communities by projecting data onto a 2 dimensional plot) on Bray–Curtis (C) are demonstrated.
L. rhamnosus L34 attenuated uremic toxin-induced injury on enterocytes, macrophages, and neutrophils
Despite several uremic toxins presented in CKD, IS is one of the major gut-derived uremic toxins contributing to CKD complications, that is correlated with several adverse effects44,46. Then, IS was used as a representative uremic toxin in vitro to test the possible effect from the secreted molecules from L34 using the L. rhamnosus condition media (LCM). In enterocytes (Caco-2 cells), IS-induced inflammation indicated by supernatant IL-8 (CXCL8) and the upregulation of inflammatory genes (CXCL8 and NF-κB) together with mucin-2 (MUC-2; a protective mechanism of enterocytes), while downregulated Occludin (OCLN; a tight junction protein), leading to the reduced transepithelial electrical resistance (TEER) (Fig. 4A-F). The incubation of IS with LCM improved these parameters, except for MUC-2 (non-difference between IS and IS with LCM), whereas LCM alone upregulated MUC-2 gene that might enhance enterocyte protection, without an alteration in other parameters (Fig. 4A-F). Similarly, LCM also attenuated IS-induced inflammation in THP-1-derived macrophages (THP-1) as detected by supernatant cytokines (TNF-α, IL-6, and IL-10), and downregulation of NF-κB and NOS-2 (Fig. 4G-K). In addition, LCM reduced IS-induced neutrophil extracellular traps (NETs), as determined by supernatant cell-free DNA and citrullinated histone 3 (CitH3), but not by nuclear morphology determined by fluorescent stanning (Fig. 4L-O). Notably, LCM alone elevated NETs only by fluorescent staining, but not by cell-free DNA and CitH3 (Fig. 4L-O), perhaps due to the different sensitivity of the test. Because the baseline level of IS in our patients was approximately 5 mg/L (0.026 mM) (Fig. 1I), this dose of IS with 4% heat-inactivated human albumin was used to test the impacts of IS at a more physiologic level (Supplementary Fig. 2). As such, a uremic IS concentration (0.026 mM) upregulated IL-8 and NF-κB but not elevated supernatant IL-8 (protein level) in Caco-2 cells, and LCM reduced IL-8 gene expression but not NF-κB and supernatant IL-8 (Supplementary Fig. 2A-C). Additionally, a uremic IS concentration elevated all cytokines in THP-1 and increased cell-free DNA and CitH3 in neutrophils (lower level than IS at 1 mM), while LCM attenuated TNF-α and IL-6 (but not IL-10) in THP-1 and reduced CitH3 (but not cell-free DNA) in neutrophils (Supplementary Fig. 2D-H). Despite a similar direction of responses against IS in the supraphysiologic and uremic concentrations, the supraphysiologic IS concentration at 1 mM (Fig. 4) induced more prominent responses than IS at 0.026 mM (a uremic concentration) (Supplementary Fig. 2) in all selected cell types, and LCM demonstrated beneficial effects. Therefore, some anti-inflammatory molecules of L34 attenuated IS-induced inflammation in enterocytes and immune cells that might be responsible for the beneficial effect of probiotics in patients (Fig. 1).
Fig. 4.
The characteristics of different cells after the 24 h incubation by the control culture media (Control) or Lacticaseibacillus condition media from L. rhamnosus L34 (LCM) or indoxyl sulfate (IS) (1 mM) or IS with LCM (IS + LCM) are demonstrated. For enterocytes (Caco-2 cells), supernatant IL-8 (A), the expression of several genes, including IL-8 (CXCL8), NF-κB, MUC-2, and occludin (OCLN) (B-E), and transepithelial electrical resistance (TEER) (F) are indicated. For THP-1-derived-macrophages (THP-1), supernatant cytokines (TNF-α, IL-6, and IL-10) (G-I) and the expression of several genes, including NF-κB and NOS2 (J, K), are used. For neutrophils, the neutrophil extracellular traps (NETs), as determined by nuclear morphology with representative fluorescent pictures (L, M), cell-free DNA (N), citrullinated histone 3 (CitH3) (O), are shown. The results were retrieved from the isolated triplicated experiments. #, p < 0.05 vs. Control, *, p < 0.05.
Possible different anti-inflammatory molecules between L. rhamnosus L34 and L. rhamnosus GG, despite a similar effect on patients with CKD
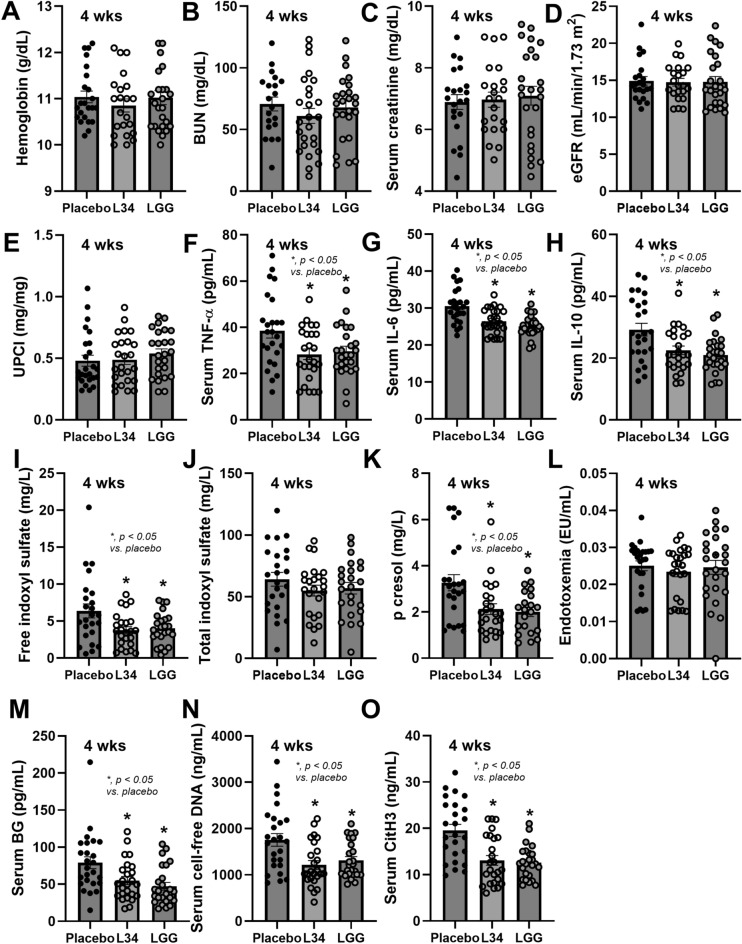
Because of the well-known anti-inflammatory property of L. rhamnosus GG (LGG) with limited data on L34, LCM from LGG and from L34 was tested in the enzyme inactivation protocol. In Caco-2 cells, amylase and lipase inactivated anti-inflammatory property of L34 (reduced supernatant IL-8), while amylase and protease inactivated the effect of LGG (Fig. 5A). These data suggested that the activation of anti-inflammation against L34 and LGG might be the glycolipids and glycoproteins, respectively. In THP-1, amylase inactivated L34 anti-inflammation (reduced supernatant IL-6) indicating exopolysaccharide as the active substance, while amylase and protease inactivated LGG anti-inflammation, indicating effects of glycoproteins (Fig. 5B). In neutrophils, amylase inactivated anti-inflammation (reduced supernatant CitH3) from both L34 and LGG (Fig. 5C), indicating exopolysaccharides of both probiotics. These data suggest some differences in the active anti-inflammatory molecules between L34 and LGG, which might differently affect patients with CKD. However, the randomization from 75 cases using placebo control, L34, and LGG (Fig. 6) demonstrated a similar attenuation of serum cytokines (TNF-α, IL-6, and IL-10), GDUTs (free IS and p-cresol, but not total IS), leaky gut (BG but not endotoxemia), and NETs (cell-free DNA and CitH3), without impacts on hemoglobin and renal functions (BUN, Cr, eGFR, and UPCI) (Fig. 7A-O). Hence, each probiotic has different anti-inflammatory molecules that might similarly affect various cells in the host, providing similar beneficial effects. The extraction of these substances or the clinical use of probiotics in advance stages of CKD is interesting.
Fig. 5.
The characteristics of enterocytes (Caco-2 cells), THP-1-derived-macrophages (THP-1), and neutrophils after 24 h incubation with indoxyl sulfate (IS) (1 mM) together with the Lacticaseibacillus condition media (LCM) from L. rhamnosus L34 (LCM from L34) or from L. rhamnosus GG (LCM from LGG) after neutralizing by several enzymes, including α-amylase (Amy), lipase (Lip), protease (Pro), and lysozyme (Lyz), or no enzyme (non) in comparison with the control media (no LCM), as demonstrated by supernatant IL-8 for Caco-2 (A), supernatant IL-6 for THP-1 (B), and citrullinated histone 3 (CitH3) for neutrophils (C), are demonstrated. The results were retrieved from the isolated triplicated experiments. #, p < 0.05 vs. No LCM, *, p < 0.05 vs. Non.
Fig. 6.
Schema of the cross-sectional analysis and the characteristics of patients with chronic kidney disease (CKD) after 4 week administration of L. rhamnosus L34 (L34) or L. rhamnosus GG (LGG) or placebo control (placebo) is demonstrated.
Fig. 7.
The characteristics of participants after the randomized control trial and the characteristics of patients with chronic kidney disease (CKD) after 4 week administration of L. rhamnosus L34 (L34) or L. rhamnosus GG (LGG) or placebo control (placebo), as indicated by hemoglobin, blood urea nitrogen (BUN), serum creatinine, estimated glomerular filtration rate (eGFR), urine protein creatinine ratio (UPCI) (A-E), serum cytokines (TNF-α, IL-6, and IL-10) (F–H), gut-derived uremic toxins (free indoxyl sulfate, total indoxyl sulfate, and p-cresol) (I-K), endotoxemia (L), serum (1→3)-beta-D-glucan (BG) (M), cell-free DNA (N), and citrullinated histone 3 (CitH3) (O) are demonstrated (n = 25/group). *, p < 0.05 vs. placebo.
Discussion
Anti-inflammatory properties and dysbiosis attenuation of L. rhamnosus L34, a pre-post experiment
The prominent inflammation in CKD is caused by several factors, including i) the increased cytokine production induced by uremic toxins, oxidative stress, and acidosis47, ii) the reduced cytokine excretion due to the impaired kidney function48, and iii) gut permeability defect (uremia-induced gut dysbiosis)32,49. Impacts of uremia during renal failure are previously demonstrated using bilateral nephrectomy mice, such as elevated inflammatory cytokines with macrophage activation and NETs50, increased half-life of injected cytokines48, enterocyte apoptosis with leaky gut (endotoxemia)32, and fecal dysbiosis49. Several randomized controlled trials on probiotic use in CKD stage 3–5 non-dialysis patients revealed the positive results on GDUTs and cytokines51, in contrast to some studies with elevated cytokine levels52, except for renal functions. Because i) uremic toxin affects all cells in the body, including the enterocytes, and ii) the administered probiotics stay inside the intestines, the anti-inflammatory effect of probiotics should be associated with uremic toxin-induced intestinal inflammation, and the inflammation worsens CKD (gut-renal axis)53. Lactobacillus is a group of bacteria in phylum Firmicutes (Bacillota) that has been reclassified into 25 new genera, and Lactobacillus rhamnosus has been changed into Lacticaseibacillus rhamnosus54. From our experience, Lactobacillus bacteria are not frequently found in fecal samples of the adult Thai population. As such, Lactobacilli were not found in the feces of 18 healthy volunteers in our previous publication23, perhaps due to the exclusion of probiotics and related health foods. Here, Lactobacillus bacteria were demonstrated in only 2 patients before the use of L34, including the patient IMH010 (L. mucosae and L. salivarius) and IMH024 (L. mucosae), and the Lactobacilli were reduced with increased Blautia spp. (the beneficial bacteria in phylum Firmicutes)55 in IMH010 and undetectable in IMH024 after L34 use. Although the L34 administration did not increase fecal Lactobacillus (fecal collection at 24 h after the last dose of L34 oral gavage) as measured by microbiome analysis, the beneficial effects of L34 were demonstrated, supporting the communication between the administered probiotics and other intestinal bacteria toward beneficial effects as previously mentioned (enhanced beneficial bacteria and short-chain fatty acid production)56.
Indeed, the pre-post experiment of L34 in CKD supported the reduced systemic inflammation (serum cytokines and NETs) partly through 2 major effects, including the attenuation of gut dysbiosis and gut permeability defect (leaky gut), as indicated by reduced p-cresol sulfate and decreased serum BG, respectively. However, the anti-inflammatory effect of L34 was not potent enough to reduce the regular anti-inflammatory parameters, including C-reactive protein (CRP) and total WBC count, perhaps due to the too short period of the probiotic administration. Notably, L34 showed a trend to reduce total form of IS and endotoxin but did not reach a significant level (p = 0.16) (Fig. 1J), possibly due to the short duration of probiotic treatment and limited number of patients. The alteration of gut microbiota by L34 was easiest to observe on the phylum level of the comparison (decreased Bacteroidota and increased Actinobacteria) with only a subtle change in the detail comparison, especially in the genus and species, which might be due to a large composition of bacteria in each phylum. For example, the Bacteroidota in one mouse is Prevotella spp., while another mouse has Bacteroides spp. without Prevotella spp., leading to the non-statistical difference in Prevotella spp. in the genus level comparison and a significant value is demonstrated only in the phylum level (Bacteroidota). Nevertheless, the altered bacterial phylum supported impacts of L34 on dysbiosis attenuation and the improved dysbiosis might reduce p-cresol sulfate. In terms of leaky gut, the presence of BG in the blood indicates the translocation of BG from the gut into the blood, possibly through the damaged intestinal tight junction of enterocyte cell death18. These BG exert inflammatory activities through the additive inflammatory responses through Dectin-1 receptor33,57. The reduction of p-cresol sulfate and BG by L34 might be responsible for the lower serum cytokines. In addition, the in vitro adverse effect of IS on enterocytes, macrophages, and neutrophils was minimized by adding probiotic condition media. Therefore, advanced CKD induces i) gut dysbiosis and circulating GDUTs3, ii) leaky gut with the presence of microbial molecules in the blood9, and iii) inflammation, resulting in the accelerated CKD progression18. Probiotics attenuate certain gut-derived uremic toxins in animal models58,59, in patients, and in the meta-analysis through several mechanisms60–63; for example, enhanced short-chain fatty acid (SCFA) production, increased beneficial bacteria, and being the competitor for food sources against pathogenic bacteria64. A vicious cycle of uremic toxins inducing gut dysbiosis and the dysbiosis further enhancing GDUTs that worsen gut leakage, systemic inflammation, and CKD progression can be disrupted by probiotics.
Anti-inflammatory effect of L. rhamnosus against uremia-induced cell injury in enterocytes, macrophages, and neutrophils
Besides dysbiosis attenuation, the anti-inflammatory properties of probiotics might synergistically attenuate CKD complications. Here, indoxyl sulfate (IS) was used as a representative toxin, promoting the production of reactive oxygen species that directly induce cell damage and cell death (apoptosis)65,66. In enterocytes (Caco-2 cells), IS reduced cell integrity (TEER) through the pro-inflammatory responses (up-regulated NF-κB transcriptional factor and increased supernatant IL-8). Meanwhile, IS activated inflammation in both macrophages and neutrophils65, and the condition media of either L34 or LGG similarly attenuated inflammation in all of these cells. To explore the active substance of probiotics, the enzyme neutralization protocol (the loss of anti-inflammatory property after the incubation with specific enzyme) was performed. In L34, the anti-inflammatory molecules against IS-induced injury in enterocytes and immune cells (macrophages and neutrophils) might be glycolipids (neutralizable by amylase and lipase) and exopolysaccharide (neutralizable only by amylase). Meanwhile, the anti-inflammatory molecules of LGG against IS-induced injury of enterocytes and macrophages might be glycoproteins, in contrast to the anti-inflammation for neutrophils might be exopolysaccharides67–69. Despite the preliminary characteristics of the enzyme neutralization experiments, both L34 and LGG demonstrated anti-inflammation in all of these cells with the possible different anti-inflammatory molecules. However, both probiotics were similarly beneficial to patients with CKD in all inflammatory markers, regardless of the possible difference in the active anti-inflammatory molecules. Notably, the significant reduced free IS and a trend to reduce total IS (non-significant difference; p = 0.20 and 0.32 between placebo vs. L34 and placebo vs. LGG, respectively) might be due to the fluctuation of protein that bound to the toxin in CKD70. Furthermore, low levels of endotoxemia were presented in all of our patients with CKD, supporting that CKD-induced leaky gut with significant renal damage71, because of the too high molecular weight of endotoxin to pass through healthy gut permeability18. Both probiotics only reduced BG, which might be due to the reaction of the limulus lysate test for endotoxin against serum proteins causing the higher measured level than the actual value72. Meanwhile, there is no interference reaction with the Fungitell assay for BG73. Further mechanistic studies to explore other protective mechanisms of probiotic-derived molecules and clinical studies with larger number of patients for a potential application in CKD are warranted.
Several limitations should be mentioned. First, several non-uremic factors might interfere with gut dysbiosis in patients with CKD, including obesity, nutrition, diabetes, and volume status74,75. Although there was no obvious obesity and malnutrition in our patients, as determined by body mass index (BMI) and serum albumin, respectively, there was some patients with clinically pitting edema and diabetes mellitus (DM) that might affect gut microbiota. Also, the direct determination of body fluid; for example, bioimpedance analysis (BIA; a non-invasive method to estimate body fat, muscle mass, and volume status)76 was not performed. Second, there was a limited number of participants with a short duration of probiotic administration. Third, IS was selected as a representative uremic toxin, despite other well-known toxins that might also potently induce proinflammation. The larger cohort studies with in vitro experiments using other uremic toxins will be interesting.
Conclusion
Both L34 and LGG similarly attenuated gut-derived uremic toxins, gut leakage, and systemic inflammation, through the protective effects on enterocytes and immune cells (macrophages and neutrophils), which might have an important role in patient with CKD in the near future.
Supplementary Information
Author contributions
A.L. designed and coordinated all the experiments, analyzed the data, and wrote and approved the manuscript. P.P. performed the experiments, performed clinical data collection, and analyzed data. T.B., W.K., P.B., and P.T. performed the experiments and performed clinical data collection. W.C., S.T., and S.U. performed clinical data collection. S.T. provided the probiotics and supervised to conception and study design.
Funding
This study was supported by the NSRF through the Program Management Unit for Competitiveness (C02F660002) with Rachadapisek Sompote Matching Fund, Chulalongkorn University.
Data availability
The data presented in this study are available on request from the corresponding author.
Declaration
Competing interests
The authors declare no competing interests.
Ethics approval
This study involves human participants and was approved by the research ethics board of the Chulalongkorn University Faculty of Medicine’s Institutional Review Board (IRB No. 0055/66). Participant gave informed consent to participate in the study before taking part.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research..
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-12768-z.
References
- 1.Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med.368(17), 1575–1584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronov, P. A. et al. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol.22(9), 1769–1776 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int.83(2), 308–315 (2013). [DOI] [PubMed] [Google Scholar]
- 4.McIntyre, C. W. et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol.6(1), 133–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijers, B. et al. Intestinal barrier function in chronic kidney disease. Toxins (Basel)10(7), 298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graboski, A. L. & Redinbo, M. R. Gut-derived protein-bound uremic toxins. Toxins (Basel).12(9), 590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, Y. et al. Indoxyl sulfate induces intestinal barrier injury through IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics10(16), 7384–7400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis, R. J. et al. Indoxyl sulfate induces apoptosis and hypertrophy in human kidney proximal tubular cells. Toxicol. Pathol.46(4), 449–459 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res.116(3), 448–455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun, C. Y., Chang, S. C. & Wu, M. S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE7(3), e34026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tungsanga, S. et al. Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules. Nephrol. Dial. Transplant.37(8), 1429–1442 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Vanholder, R. et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol.25(9), 1897–1907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu, I. W. et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant.26(3), 938–947 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meijers, B. K. et al. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant.25(1), 219–224 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Wang, I. K. et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: A randomised, double-blind, placebo-controlled trial. Benef. Microbes.6(4), 423–430 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Rossi, M. et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol.11(2), 223–231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guida, B. et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis.24(9), 1043–1049 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Amornphimoltham, P. et al. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig. Dis. Sci.64(9), 2416–2428 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Andrade-Oliveira, V. et al. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol.10, 1192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau, W. L. & Vaziri, N. D. The Leaky Gut and Altered Microbiome in Chronic Kidney Disease. J. Ren. Nutr.27(6), 458–461 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Kang, G. G. et al. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH. Science26(1), 105905 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y. et al. Stress triggers gut dysbiosis via CRH-CRHR1-mitochondria pathway. NPJ Biofilms Microbiomes10(1), 93 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panpetch, W. et al. Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin. PLoS ONE16(12), e0261189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boonma, P. et al. Draft genome sequences and description of Lactobacillus rhamnosus strains L31, L34, and L35. Stand Genomic Sci.9(3), 744–754 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chancharoenthana, W. et al. Cilostazol attenuates intimal hyperplasia in a mouse model of chronic kidney disease. PLoS ONE12(12), e0187872 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panpetch, W. et al. Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut. Infect. Immun.86(1), 10–1128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panpetch, W. et al. Corrigendum: Candida Administration Worsens Cecal Ligation and Puncture-Induced Sepsis in Obese Mice Through Gut Dysbiosis Enhanced Systemic Inflammation, Impact of Pathogen-Associated Molecules From Gut Translocation and Saturated Fatty Acid. Front. Immunol.11, 613095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panpetch, W. et al. Oral Candida administration in a Clostridium difficile mouse model worsens disease severity but is attenuated by Bifidobacterium. PLoS ONE14(1), e0210798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrassy, K. M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int.84(3), 622–623 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Leelahavanichkul, A. et al. Evaluation of gastrointestinal leakage using serum (1→3)-β-D-glucan in a Clostridium difficile murine model. FEMS Microbiol. Lett.363(18), fnw204 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Panpetch, W. et al. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes11(3), 465–480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panpetch, W. et al. Candida Administration Worsens Uremia-Induced Gut Leakage in Bilateral Nephrectomy Mice, an Impact of Gut Fungi and Organismal Molecules in Uremia. mSystems.6(1), 10–1128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tungsanga, S. et al. Candida Administration in 5/6 Nephrectomized Mice Enhanced Fibrosis in Internal Organs: An Impact of Lipopolysaccharide and (1→3)-β-D-Glucan from Leaky Gut. Int. J. Mol. Sci.23(24), 15987 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irinyi, L. et al. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database–the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med. Mycol.53(4), 313–337 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Saisorn, W. et al. Extracellular traps in peripheral blood mononuclear cell fraction in childhood-onset systemic lupus erythematosus. Sci. Rep.14(1), 23177 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sae-Khow, K., Charoensappakit, A. & Leelahavanichkul, A. Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia. Int. J. Mol. Sci.25(19), 10651 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saithong, S. et al. Candida Administration Worsens Neutrophil Extracellular Traps in Renal Ischemia Reperfusion Injury Mice: An Impact of Gut Fungi on Acute Kidney Injury. J. Innate. Immun.14(5), 502–517 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Issara-Amphorn, J. et al. Syk inhibitor attenuates inflammation in lupus mice from FcgRIIb deficiency but not in pristane induction: The influence of lupus pathogenesis on the therapeutic effect. Lupus29(10), 1248–1262 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Putt, K. K. et al. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct.8(1), 406–414 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Lee, H. W. et al. Uremic serum damages endothelium by provoking excessive neutrophil extracellular trap formation. Sci. Rep.11(1), 21439 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wexler, H. M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev.20(4), 593–621 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binda, C. et al. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis.50(5), 421–428 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Rinninella, E. et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms7(1), 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan, C. K. I. et al. Symbiotic Firmicutes establish mutualism with the host via innate tolerance and resistance to control systemic immunity. Cell Host Microbe31(9), 1433-1449.e9 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Zhang, S. et al. The role of the microbiome in diabetes mellitus. Diabetes Res. Clin. Pract.172, 108645 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Leong, S. C. & Sirich, T. L. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins (Basel)8(12), 358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mihai, S. et al. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res.2018, 2180373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leelahavanichkul, A. et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int.80(11), 1198–1211 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chancharoenthana, W. et al. Lacticaseibacilli attenuated fecal dysbiosis and metabolome changes in Candida-administered bilateral nephrectomy mice. Front. Immunol.14, 1131447 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suksawad, N. et al. Cyclic GMP-AMP Synthase (cGAS) Deletion Reduces Severity in Bilateral Nephrectomy Mice through Changes in Neutrophil Extracellular Traps and Mitochondrial Respiration. Biomedicines11(4), 1208 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian, N. et al. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients14(19), 4044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia, L. et al. Efficacy of Probiotics Supplementation On Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press Res.43(5), 1623–1635 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Stavropoulou, E. et al. Focus on the Gut-Kidney Axis in Health and Disease. Front. Med. (Lausanne)7, 620102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng, J. et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol.70(4), 2782–2858 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Verstraeten, S. et al. Description of a Newly Isolated Blautia faecis Strain and Its Benefit in Mouse Models of Post-Influenza Secondary Enteric and Pulmonary Infections. Nutrients14(7), 1478 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ondee, T. et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep.11(1), 6367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tungsanga, S. et al. Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1→3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34. Int. J. Mol. Sci.23(5), 2511 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ranganathan, N. et al. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci. World J.5, 652–660 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ranganathan, N. et al. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. Asaio J.52(1), 70–79 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Ranganathan, N. et al. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin.25(8), 1919–1930 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Ranganathan, N. et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther.27(9), 634–647 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Dehghani, H. et al. Synbiotic Supplementations for Azotemia in Patients With Chronic Kidney Disease: A Randomized Controlled Trial. Iran J. Kidney Dis.10(6), 351–357 (2016). [PubMed] [Google Scholar]
- 63.Poesen, R. et al. The Influence of Prebiotic Arabinoxylan Oligosaccharides on Microbiota Derived Uremic Retention Solutes in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. PLoS ONE11(4), e0153893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tao, S. et al. Effects of probiotic supplements on the progression of chronic kidney disease: A meta-analysis. Nephrology (Carlton)24(11), 1122–1130 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Mutsaers, H. A. et al. Chronic Kidney Disease and Fibrosis: The Role of Uremic Retention Solutes. Front. Med. (Lausanne)2, 60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin, Y. T. et al. Indoxyl Sulfate Induces Apoptosis Through Oxidative Stress and Mitogen-Activated Protein Kinase Signaling Pathway Inhibition in Human Astrocytes. J. Clin. Med.8(2), 191 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oleksy, M. & Klewicka, E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr.58(3), 450–462 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Paściak, M. et al. Lactobacillus johnsonii glycolipids, their structure and immunoreactivity with sera from inflammatory bowel disease patients. Microb. Biotechnol.10(2), 456–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malamud, M. et al. S-Layer Glycoprotein From Lactobacillus kefiri Exerts Its Immunostimulatory Activity Through Glycan Recognition by Mincle. Front. Immunol.10, 1422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liabeuf, S., Drüeke, T. B. & Massy, Z. A. Protein-bound uremic toxins: New insight from clinical studies. Toxins (Basel)3(7), 911–919 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boonhai, S. et al. TMAO reductase, a biomarker for gut permeability defect induced inflammation, in mouse model of chronic kidney disease and dextran sulfate solution-induced mucositis. As. Pac. J. Allergy Immunol.41(2), 168–178 (2023). [DOI] [PubMed] [Google Scholar]
- 72.Harm, S. et al. Heparin enables the reliable detection of endotoxin in human serum samples using the Limulus amebocyte lysate assay. Sci. Rep.14(1), 2410 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leelahavanichkul, A. et al. Gastrointestinal Leakage Detected by Serum (1→3)-β-D-Glucan in Mouse Models and a Pilot Study in Patients with Sepsis. Shock46(5), 506–518 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Zhang, L. et al. Gut microbiota and therapy for obesity and type 2 diabetes. Front. Endocrinol. (Lausanne)15, 1333778 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li, X., Watanabe, K. & Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol.8, 1882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chongthanakorn, K. et al. Effective determination of dry weight by intradialytic bioimpedance analysis in hemodialysis. Blood Purif.27(3), 235–241 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.