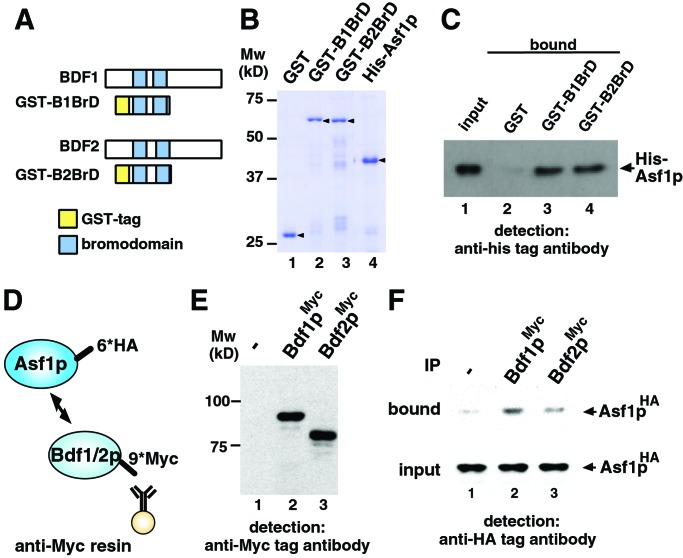
Figure 2.
Interaction between Asf1p and Bdf1/2p bromodomains. (A) Representation of the bromodomains in Bdf1p and Bdf2p. Schematic drawings of GST-tagged bromodomains of Bdf1p and Bdf2p also are shown as GST-B1BrD and GST-B2BrD, respectively. (B) Purified recombinant proteins used in the GST pull-down assay (arrowhead). Approximately 0.2 μg of each protein was separated by SDS/PAGE and stained with Coomassie brilliant blue. Mw, molecular mass markers. (C) Direct interaction between Asf1p and bromodomains of Bdf1p and Bdf2p by GST pull-down assay. GST-tagged proteins (8.0 × 10−12 mol) and His-tagged Asf1p (2.5 × 10−11 mol) were mixed. One percent of the input (lane 1) and 30% of the pull-down material (lanes 2–4) were separated by SDS/PAGE, and His-tagged Asf1p was detected by immunoblot analysis using anti-His-tag antibody. (D) Schematic drawing of immunoprecipitation analysis. 6×HA epitope (6*HA) and 9×Myc epitope (9*Myc) are tagged at the C terminus of Asf1p and Bdf1p/Bdf2p, respectively. Myc-tagged Bdf1p or Bdf2p was immunoprecipitated with anti-Myc affinity resin. (E) Expression of Myc-tagged proteins. WCE of yeast strain expressing Myc-tagged Bdf1p (lane 2) or Bdf2p (lane 3) was separated by SDS/PAGE, and Myc-tagged proteins were detected by Western immunoblot analysis using anti-Myc antibody. No signal was detected in the WCE from control strain in which no protein is Myc-tagged (lane 1). (F) Interaction between Asf1p and Bdf1p/Bdf2p in yeast cells. HA-tagged Asf1p in bound fraction was detected by immunoblot analysis using anti-HA tag antibody (bound). A 4% volume of each WCE also was immunoblotted (input) to confirm that an equal amount of HA-tagged Asf1p is expressed in Myc-tagged Bdf1p- or Bdf2p-expressing (lanes 2 and 3, respectively) or control (lane 1) strains. IP, immunoprecipitation.