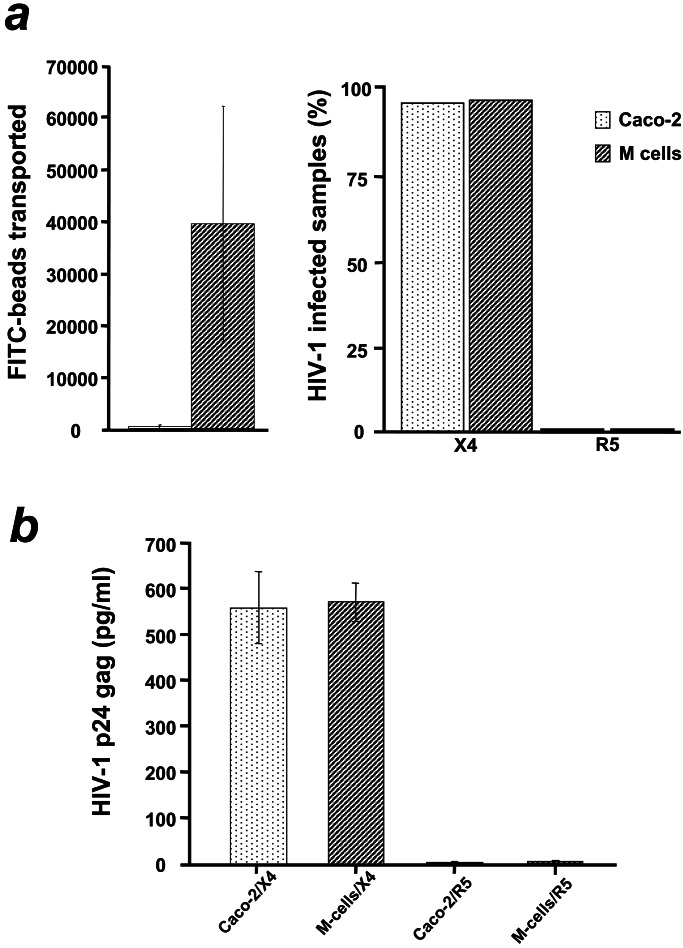
Figure 3.
Receptor-mediated transcytosis accounts for early HIV-1 penetration of the epithelial monolayers. (a Left) FITC-coupled beads added on the apical medium of both Caco-2 and M cell monolayers were transported within 1 h across converted but not across normal Caco-2 monolayers. Bars represent the average number of beads measured by cytofluorometry in the medium of the lower chamber from four independent experiments. Monolayers with low transepithelial resistance (TER), high 3H-inulin diffusion or high number of beads transported after the first 15 min of incubation were discarded. (a Right) The same monolayers used to monitor transcytosis were exposed apically to X4 or R5 HIV-1 and the underlying target CD4+ T cells or monocytes were recovered and tested for infection by PCR. No infection of target monocytes (0% infected samples) was observed in converted monolayers despite efficient transport of fluorescent beads. In contrast CD4+ T cells under normal monolayers that were totally impermeable to latex beads were infected. Mean values of four independent experiments (12 monolayers per experiment) expressed as percentage of infected samples (pool of mononuclear target cells underneath converted or not Caco-2 monolayers) with HIV-1. (b) HIV-1 p24 gag antigen was captured by ELISA in the basolateral medium, as soon as 30 min after apical infection of normal (n = 12) or converted Caco-2 (n = 12) monolayers, only when X4 but not R5 HIV-1 was added. Bars represent the average p24 gag concentration in picograms per milliliter in two independent experiments. Equal concentration of X4 and R5 HIV-1 was added apically. The maximum threshold of the detection system is 600 pg/ml; 10 pg of p24 correspond to 105 particles.