Figure 2.
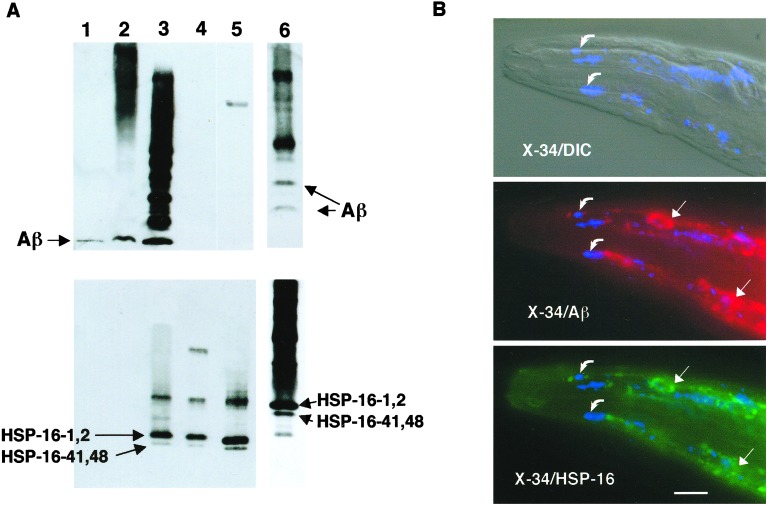
Association of Aβ with HSP-16. (A) Coimmunoprecipitation of Aβ and HSP-16. Lysates from CL2006 were immunoprecipitated with either mAb 4G8 (lanes 1–5) or anti-HSP-16 antibody (lane 6) and fractionated along with control preparations on a Tris-Bicine/SDS polyacrylamide gel, and then blotted and sequentially probed with 4G8 (Upper) and anti-HSP16 antibody (Lower). Lane 1, synthetic Aβ1–42; lane 2, total CL2006 lysate; lane 3, CL2006 4G8 immunoprecipitate; lane 4, concentrated post-4G8-IP lysate; lane 5, heat-shocked wild-type animals; lane 6, CL2006 anti-HSP-16 immunoprecipitate (run on a separate gel, resulting in different mobilities than lanes 1–5). Note both Aβ and HSP-16 proteins detected in both immunoprecipitates (lanes 3 and 6). (B) Immunohistochemical localization of Aβ, HSP-16, and amyloid dye-reactive deposits in CL2006. CL2006 animals were vitally stained with the amyloid-specific dye X-34 and then fixed, permeabilized, and probed with 4G8 and anti-HSP-16 antibody. (Top) Digitally fused differential contrast (DIC)/shortwave epifluorescence (X-34) image; (Middle) fused X-34/4G8 epifluorescence image; (Bottom) fused X-34/anti-HSP-16 epifluorescence image. (All epifluorescence images are false color.) Note nearly identical patterns of Aβ- and HSP-16-reactive deposits (straight arrows), which do not generally overlap with X-34-reactive amyloid deposits (curved arrows). (Bar = 25 μm.)