Abstract
Blood-flow interactions with the vascular endothelium represents a specialized example of mechanical regulation of cell function that has important physiological and pathophysiological cardiovascular consequences. Yet, the mechanisms of mechanostransduction are not understood fully. This study shows that shear stress induces a rapid induction as well as nuclear translocation of the vascular endothelial growth factor (VEGF) receptor 2 and promotes the binding of the VEGF receptor 2 and the adherens junction molecules, VE-cadherin and β-catenin, to the endothelial cytoskeleton. These changes are accompanied by the formation of a complex containing the VEGF receptor 2–VE-cadherin–β-catenin. In endothelial cells lacking VE-cadherin, shear stress did not augment nuclear translocation of the VEGF receptor 2 and phosphorylation of Akt1 and P38 as well as transcriptional induction of a reporter gene regulated by a shear stress-responsive promoter. These results suggest that VEGF receptor 2 and the adherens junction act as shear-stress cotransducers, mediating the transduction of shear-stress signals into vascular endothelial cells.
As blood flows through the arteries, it imparts physical forces to the vascular wall that regulate a number of important physiological responses in blood vessels and also are implicated in the development of arterial wall pathologies. It now is well established that the primary role of hemodynamic forces (mainly shear stress, a frictional force acting at the interface between the flowing blood and the vessel wall), acting through the endothelium, is to cause chronic restructuring of blood vessels (1–6) as well as to initiate blood-vessel formation through a process termed arteriogenesis (7, 8). Several in vivo observations suggest that hemodynamic forces modulate endothelial structure and function, which include increased permeability of macromolecules, lipoproteins accumulation, and endothelial cell damage and repair near branch points and bifurcations (2, 4, 6). More conclusive evidence for the direct effect of hemodynamic forces on endothelial structure and function has come from in vitro studies in which cultured monolayers have been subjected to defined hemodynamic forces in well controlled model systems. Shear stress-induced structural and functional changes in the vascular endothelium raise the questions “how are mechanical forces transduced by endothelial cells into biological response?” and “is there a shear-stress receptor?” Mechanistic studies of shear-stress responsiveness have focused mainly on the potential role of ion channels, tyrosine kinase receptors, G proteins, activation of polyphosphoinositide cascade, and modulation of protein function through the calcium and phosphorylation mechanism. The endothelial cytoskeleton also has been considered a candidate for shear-stress transduction because of its fast and dramatic response to shear. It was shown recently that two types of integrins, αvβ3 and α5β1, as well as additional focal adhesion components such as FAK and Src, may serve as mechanotransducers in endothelial cells exposed to shear stress (4, 9).
The main receptors involved in signal transduction cascade in response to vascular endothelial (VE) growth factor (VEGF) comprise a family of closely related receptor tyrosine kinases consisting of three members: the VEGF receptors (VEGFRs) 1, 2, and 3. VEGFR2 seems to mediate the major growth and permeability actions of VEGF, and its deletion in mice is lethal (10, 11). Recently, early activation (phosphorylation) of VEGFR2 was demonstrated in endothelial cells exposed to shear stress (12). VEGFR2 phosphorylation was accompanied by VEGFR2 membranal clustering and transient binding to the adaptor protein Shc.
The endothelial cell–cell adhesion site also was suggested as a potential site for mechanosensing. A rapid tyrosine phosphorylation of platelet endothelial cell adhesion molecule 1 (PECAM-1) was observed in response to flow, and was accompanied by the binding of PECAM-1 to the phosphatase SH2 and the accumulation of signaling molecules near the junction (13).
VE-cadherin is the major adhesive protein of the adherens junction and is specific for vascular endothelial cells. It can transfer intracellular information by interacting with the cytoskeleton via several anchoring molecules (among them is β-catenin), and its expression is required for vascular integrity (14). Deletion or cytosolic truncation of VE-cadherin impairs remodeling and maturation of the vascular network, and on the cellular level it abolishes transmission of intracellular signal via VEGFR2 (15). VEGF induces tyrosine phosphorylation of VE-cadherin, β-catenin, plakoglobin, and p-120, which is mediated by VEGFR2 (16). Recently VEGFR2 was shown to form a complex with VE-cadherin, β-catenin, and phosphatidylinositol 3-kinase that leads to the phosphorylation of the survival signal Akt1 (phospho-kinase B) and the induction of BclII in vascular endothelial cells (15). The adherens junction has been shown to play a role in the adaptation of vascular endothelial cells to long intervals of shear stress (17, 18), but its role in mechanosensing has not been studied. The present study demonstrates that short intervals of shear stress stimulate the formation of the VEGFR2–VE-cadherin–β-catenin complex in vascular endothelial cells, and that the complex plays a role in transducing shear stress-dependent signals into the endothelium. We also suggest that VEGFR2 mediates the communication between several shear-stress receptors and the orchestration of shear stress-mediated cellular signals.
Materials and Methods
Cell Culture.
Primary bovine aortic endothelial cells (BAECs) were prepared as described (19) and grown to confluence in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum. Twenty-four hours before each experiment the cultures were serum-starved with medium containing 0.5% calf serum. Primary human umbilical vein endothelial cells were prepared as described (20) and grown in M-199 medium with Hanks' salt base containing 20% FCS, 50 μg/ml endothelial mitogen, and 20 units/ml heparin. Twenty-four hours before each experiment the cultures were serum-starved with medium containing 5% FCS without mitogens and heparin. Endothelial VE-cadherin−/− (100) and VE-cadherin+/+ (100VE) cells were prepared as described (15) and grown in DMEM containing 20% FCS, 50 μg/ml endothelial mitogen, and 20 units/ml heparin. Twenty-four hours before each experiment the cultures were serum-starved with medium containing 5% FCS without mitogens and heparin.
Transfection Procedure.
BAECs or 100 and 100VE cultures were electroporated by using the Electro cell manipulator (ECM600, BTX, Genetronics, San Diego, CA). In brief, 24 h after their passage the cells were collected into DMEM/10% FCS and electroporated following the conditions supplied by the manufacturer with a vector containing the firefly luciferase regulated by a promoter containing the platelet-derived growth factor (PDGF)-A/shear stress response element (SSRE) (21) and a control vector of the Renilla luciferase regulated by a minimal promoter. One day later the cells were washed and transferred to shear-stress plates. At the end of each experiment the cells were collected, lysed, and analyzed for dual luciferase activity by using the dual-luciferase kit (Promega) and a luminometer (TD 20/20 Turner Designs, Sunnyvale, CA).
Shear-Stress Experiments.
Cells were plated on gelatin-treated polystyrene plates for protein-extraction experiments and on polystyrene coverslips for immunofluorescence experiments. Cells were subjected to laminar shear stress (LSS) of 10 dynes/cm2 (1 dyne = 10 μN) for changing periods of time using the cone and plate apparatus as described (19). All experiments were repeated at least four times.
Preparation of Whole-Cell Extracts.
Cells were washed three times in PBS containing Ca2+, Mg2+, and 1 mM Na3Vo4, harvested, and lysed in lysis buffer (20 mM Tris, pH 7.5/150 mM NaCl/1 mM Na3Vo4/5 mM EDTA/1% Triton X-100/10% glycerol/1 mM DTT/1 mM PMSF/1 μg/ml leupeptin).
Preparation of Cytosolic and Nuclear Extracts.
Cytosolic and nuclear proteins from BAECs were obtained as described (22).
Preparation of Triton X-100-Soluble and Triton X-100-Insoluble Protein Extracts.
Triton X-100-soluble and -insoluble protein extracts were prepared as described (17).
Immunofluorescence Staining.
Cells were washed three times in PBS, fixed in 2% paraformaldehyde for 20 min, and permeabilized in 2% paraformaldehyde containing 0.2% Triton X-100. Cells then were blocked in PBS containing 3% BSA, 10% rabbit serum, and 0.1% glycine and incubated with the primary antibody in PBS with 2% rabbit serum followed by incubation with FITC-conjugated or tetramethylrhodamine B isothiocyanate-conjugated secondary antibody. All procedures were carried out by using PBS containing Ca2+ and Mg2+. Image processing was carried out on a Zeiss inverted microscope (Axiovert B5) and IMAGE PRO (plus 4.5) software.
Western Blot Analysis.
Protein extracts were subjected to electrophoresis on SDS-polyacrylamide gels and electrotransferred onto nitrocellulose membranes. Membranes were blocked for 1 h in 5% nonfat dry milk in Tris-buffered saline (TBS)/0.05% Tween 20 except when anti-Akt and anti-phospho-Akt antibodies were used, for which blocking was carried out in 5% BSA in TBS/0.05% Tween 20. Blots were incubated overnight at 4°C with primary antibodies (0.5 μg/ml) in their blocking solution followed by 1-h incubation with horseradish peroxidase-conjugated protein A/G (1:5,000 dilution, Pierce) and detected by a chemiluminescence kit (LumiGlo, Cell Signaling, Beverly, MA).
Antibodies.
Anti-Flk-1 (sc-1158), anti-VE-cadherin (sc-6458), anti-β-catenin (sc7199), and anti-γ-catenin (plakoglobin, sc-7900) were purchased from Santa Cruz Biotechnology. Anti-Akt (9272) and phospho-Akt (9271), anti-P38 (9212), and phospho-P38 (9212s) were purchased from Cell Signaling.
Data Quantification and Statistical Analysis.
Immunoblots were quantified by using a Vilber Lourmat densitometer (BIO CAP-1 software). All values are reported as mean ± SD. Statistical significance was calculated by using an ANOVA two-tailed t test and set as P < 0.05.
Results
Rapid Response of VEGFR2 to Shear Stress.
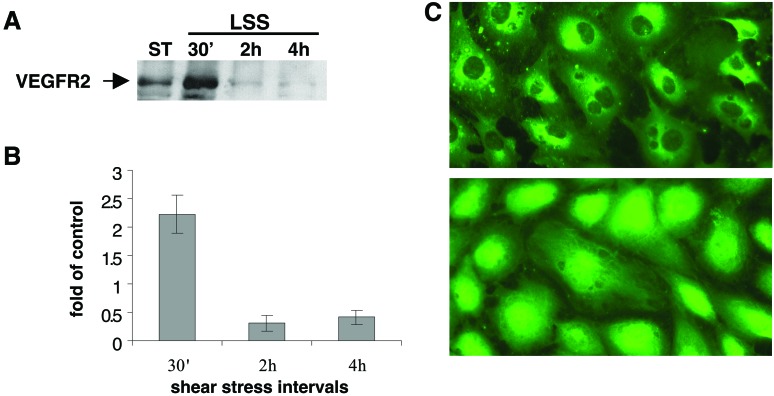
To test the regulation of VEGFR2 by shear stress, BAECs were exposed to physiological levels of shear stress (10 dynes/cm2), and the protein levels of VEGFR2 in these cells were tested. Rapid induction (30 min) was observed in whole-cell extracts prepared from endothelial cells exposed to shear stress in comparison to cells grown under static conditions (Fig. 1 A and B). Interestingly, VEGF-A levels were unaffected by shear stress (data not shown). The induction of VEGFR2 by shear stress diminished several hours after the onset of flow, suggesting that this transient response is affected by shear-stress changes rather than chronic shear stress. To our surprise shear stress regulated not only the levels of VEGFR2 but also its cellular localization. Both immunohistochemical studies (Fig. 1C) and Western Blot analysis of BAEC cytosolic and nuclear fractions suggested that as early as 2 min after the onset of flow, VEGFR2 translocated into the nucleus. A similar translocation could be observed in endothelial cells treated with VEGF for several hours (ref. 23 and N.R., unpublished results). Although the biological function of the nuclear VEGFR2 is yet to be defined, nuclear translocation of additional tyrosine kinase receptors such as fibroblast growth factor and epidermal growth factor was demonstrated also, a translocation that was accompanied by transcriptional activity of the receptors (24, 25).
Figure 1.
VEGFR2 expression and cellular localization are regulated by shear stress. BAECs were grown under static conditions or exposed to laminar shear stress (10 dynes/cm2) for various time intervals. (A) The expression of VEGFR2 was tested in whole-cell extracts. (B) Quantification of VEGFR2 expression. (C) The cells were fixed with 2% paraformaldehyde containing 0.5% Triton X-100 following 10-min exposure to LSS and immunostained. Images were taken on a Zeiss inverted microscope (×400) by using IMAGE PRO software.
Rapid Response of the Adherens Junction to Shear Stress.
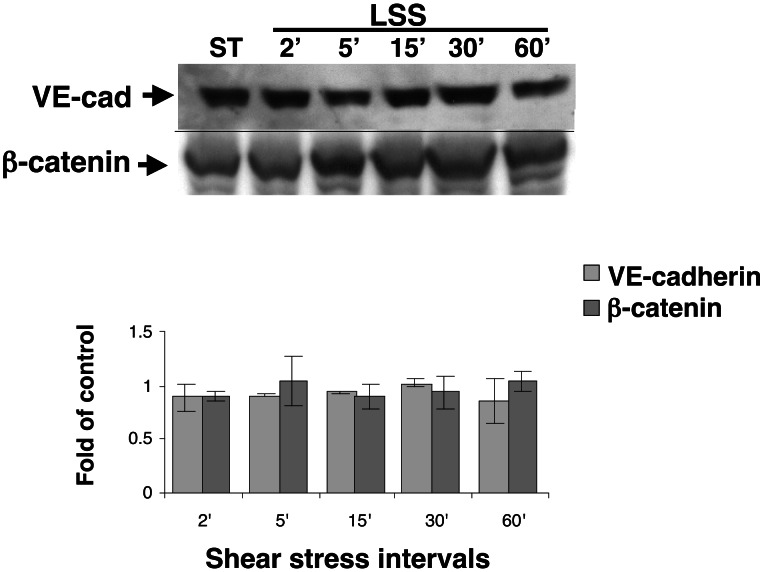
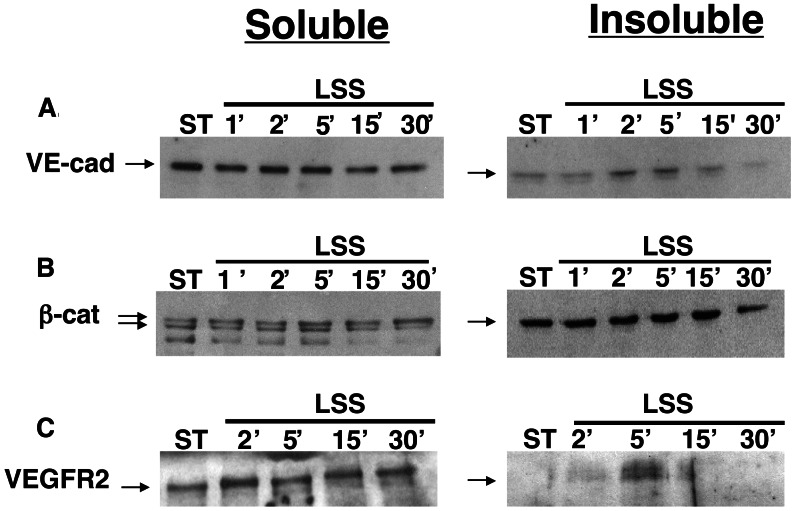
Recent reports demonstrated changes in cellular localization of the adherens junctions components, VE-cadherin, and β-catenin in endothelial cells exposed to prolonged shear stress (8–24 h) and suggested a potential role for these molecules in the morphological adaptation of endothelial cells to flow (17, 18). We hypothesized that the adherens junction may serve as a shear-stress sensor and thus undergo fast changes in response to flow. To test this hypothesis, endothelial cells were exposed to short intervals of shear stress, and the cellular localization of the two molecules was studied. Although the expression level of these molecules was unchanged in cells exposed to short intervals (minutes to hours) of shear stress (Fig. 2), changes in the cellular localization of VE-cadherin and β-catenin after shear stress were detected by immunofluorescent costaining. In endothelial cells exposed to 5 min of shear stress, VE-cadherin and β-catenin accumulated and colocalized at the intercellular junctions (data not shown). To test the binding of VE-cadherin and β-catenin to the endothelial cytoskeleton, endothelial cells were extracted into Triton X-100-soluble and -insoluble fractions. A rapid but transient increase was observed in the cytoskeletal (Triton-insoluble)-bound fraction of VE-cadherin minutes (2′–5′) after the onset of flow (Fig. 3A), whereas the levels of β-catenin in both fractions were not affected by shear stress (Fig. 3B). Interestingly, VEGFR2 also became associated with the cytoskeleton shortly (5′) after the onset of flow (Fig. 3C) in a time interval similar to the binding of VE-cadherin to the cytoskeleton.
Figure 2.
The cellular localization but not the expression of VE-cadherin (VE-cad) and β-catenin expression is unaffected by shear stress. BAECs were grown under static conditions or exposed to LSS (10 dynes/cm2) for various time intervals. The expression of VE-cadherin and β-catenin was tested in whole-cell extracts by using immunoblotting and compared with the expression of a reference gene, Tie 2, the expression of which is unaffected by shear stress (N.R., unpublished observation). The data are presented as fold of control, mean ± SD (n = 4).
Figure 3.
Rapid and transient attachment of VE-cadherin and VEGFR2 to the endothelial cytoskeleton in endothelial cells exposed to shear stress. BAECs were grown under static conditions or exposed to LSS (10 dynes/cm2) for various time intervals. Cytoskeleton-free (Triton X-100-soluble) and bound (Triton X-100-insoluble) fractions were prepared and tested for the presence of VE-cadherin (A), β-catenin (B), and VEGFR2 (C) by using immunoblotting. The data shown is a representative experiment (n = 5). ST, static BAEC control cultures.
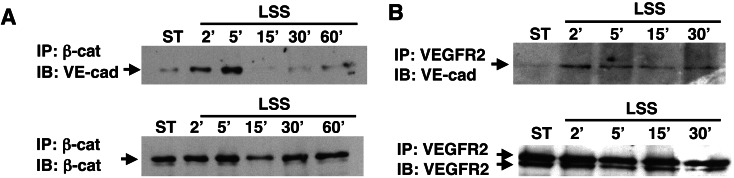
Because VE-cadherin total-protein levels are unchanged in response to shear stress (Fig. 2), the movement of VE-cadherin from the soluble to the insoluble fraction could be explained by increased binding to one of its cytoskeleton-anchoring proteins. To test this possibility, coimmunoprecipitation assays were performed by using either β-catenin or γ-catenin (plakoglobin) antibodies and cellular extracts derived from static or shear-stress cells. These experiments revealed that the binding of VE-cadherin to plakoglobin was high under static conditions but not affected by the flow (data not shown). In contrast, the binding of VE-cadherin to β-catenin was minimal under static conditions, but a rapid and transient increase occurred in this binding after exposure of endothelial cells to shear stress (Fig. 4A). The binding of VE-cadherin to β-catenin overlapped the binding of VE-cadherin to the endothelial cytoskeleton as well as their colocalization at the cellular junction.
Figure 4.
Rapid and transient attachment of VE-cadherin to β-catenin and VEGFR2 in endothelial cells exposed to shear stress. BAECs were grown under static conditions or exposed to LSS (10 dynes/cm2) for various time intervals. (A) Coimmunoprecipitation (IP) was performed to whole-cell extracts by using an antibody to β-catenin. Precipitated samples were tested for the presence of VE-cadherin (VE-cad) or β-catenin by using immunoblotting (IB). (B) Coimmunoprecipitation was performed with whole-cell extracts by using an antibody to VEGFR2. Precipitated samples were tested for the presence of VE-cadherin or VEGFR2 by using immunoblotting. ST, static BAEC control cultures.
Rapid and Transient Binding of VEGFR2 to the Adherens Junctions After Exposure of the Cells to Shear Stress.
Recent studies demonstrated that after treatment of endothelial cells with VEGF-A, a multimember complex is formed containing VEGFR2, VE-cadherin, and β-catenin (15, 26). In cultured cells this complex was responsible for the induction of survival signals, whereas in vivo it played a major role in new blood-vessel formation. The binding of VEGFR2 to the endothelial cytoskeleton after shear stress (Fig. 3C) raised the possibility that shear stress similar to VEGF induces the formation of the complex VEGFR2–VE-cadherin–β-catenin. Coimmunoprecipitation studies with either β-catenin (data not shown) or VEGFR2 (Fig. 4B) antibodies, revealed a rapid and transient binding of VEGFR2 to VE-cadherin and β-catenin, which overlapped the formation of the complex VE-cadherin–β-catenin and the binding of VEGFR2 to the endothelial cytoskeleton. Recently, shear stress was demonstrated to induce a rapid (1–2 min) activation (tyrosine phosphorylation) of VEGFR2 (12). Thus, after the exposure of endothelial cells to shear stress, VEGFR2 is activated rapidly, binds to the endothelial cytoskeleton, and forms a transient multimember complex with the adherens junction components.
The Complex VE-Cadherin–β-Catenin–VEGFR2 Plays a Role in Shear-Stress Signaling.
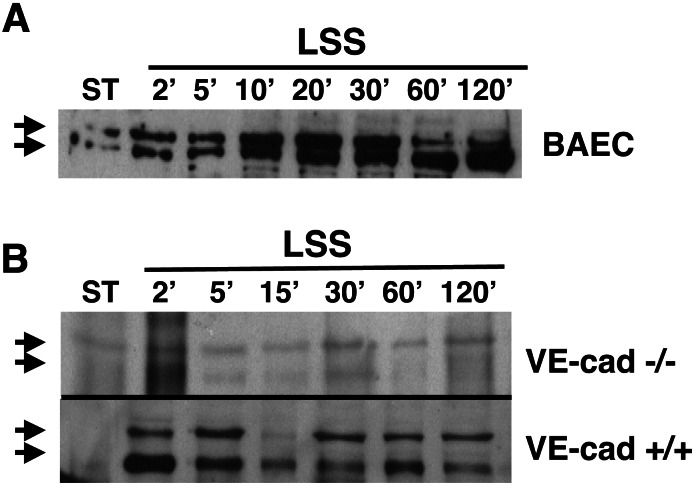
Several candidates including tyrosine kinase receptors, integrins, ion channels, and caveolae were implicated as potential shear-stress “receptors” undergoing rapid changes in endothelial cultures exposed to shear stress (4). The shear stress-mediated rapid changes in the adherens junction and VEGFR2 cellular localization demonstrated here pointed at this complex as a potential shear-stress transducer. To test the role of the complex as a shear-stress transducer, two embryonic endothelial cell lines were used (15). In the first line the VE-cadherin gene was knocked out (VE-cad−/−), and in the second the VE-cadherin gene was stably retransfected (VE-cad+/+). Several shear stress-induced signaling events were tested in these cells after their exposure to shear stress: nuclear translocation of VEGFR2 (Fig. 5), activation of the serine kinases Akt1 (phospho-kinase B) and P38 (Fig. 6 A and B), and transcriptional induction of a reporter gene regulated by a hybrid promoter containing the PDGF-A/SSRE (Fig. 7). VEGFR2 nuclear translocation in response to shear stress was observed in both arterial endothelial cells and the VE-cad+/+ line (Fig. 5 A and B) but did not occur in VE-cad−/− cells (Fig. 5B).
Figure 5.
Nuclear translocation of VEGFR2 mediated by shear stress does not occur in endothelial cells lacking the VE-cadherin gene. BAECs (A), endothelial cells null for VE-cadherin (VE-cad −/−), and the same cells stably retransfected with VE-cadherin (VE-cad +/+, B) were grown under static conditions or exposed to LSS (10 dynes/cm2) for various time intervals. Nuclear fractions were prepared and tested for the presence of VEGFR2 (double arrow) by using immunoblotting. Note the rapid (2′) nuclear translocation of VEGFR2 after shear stress in BAECs and VE-cadherin +/+ cells.
Figure 6.
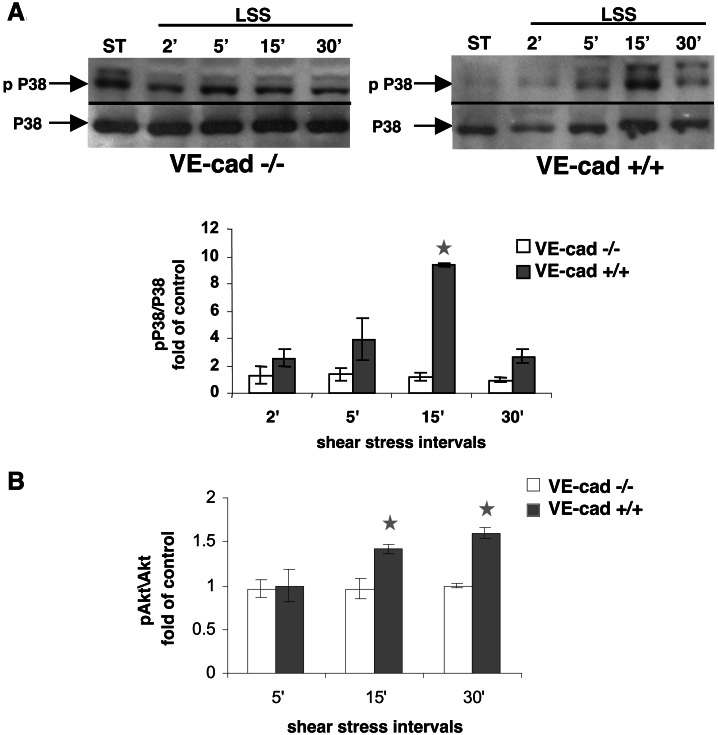
Shear stress-mediated activation (phosphorylation) of p38 does not occur in endothelial cells lacking the VE-cadherin gene. (A) Endothelial cells null for VE-cadherin (VE-cad −/−) and the same cells stably retransfected with VE-cadherin (VE-cad +/+) were grown under static conditions or exposed to LSS (10 dynes/cm2) for various time intervals. Whole-cell extracts were tested for P38 expression or phosphorylation (pP38). (B) Endothelial cells null for VE-cadherin (VE-cad −/−) and the same cells stably retransfected with VE-cadherin (VE-cad +/+) were grown under static conditions or exposed to LSS (10 dynes/cm2) for various time intervals. The expression and activation of Akt were tested and are presented as the ratio of Akt to phosphorylated Akt (mean ± SD, n = 4).
Figure 7.
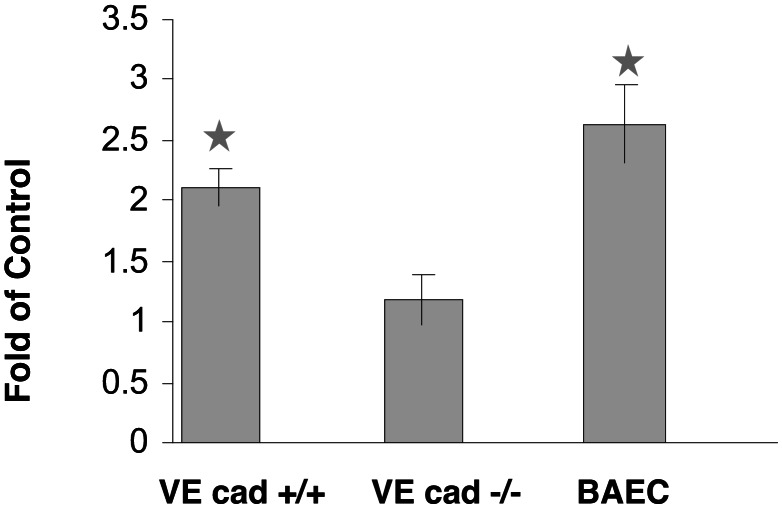
Transcriptional induction of a PDGF-A/SSRE-luciferase vector does not occur in endothelial cells lacking the VE-cadherin gene. BAEC cultures, endothelial cells null for VE-cadherin (VE-cad −/−), and the same cells stably retransfected with VE-cadherin (VE-cad +/+) were cotransfected with a vector containing the PDGF-A/SSRE regulating the expression of a firefly luciferase reporter gene and a Renilla luciferase regulated by a minimal promoter. The transfected cells were incubated under static conditions or exposed to LSS (10 dynes/cm2, 60 min), and the expression of the firefly luciferase was normalized to the expression of the Renilla luciferase. Statistical analysis was carried out on five independent experiments.
P38 (mitogen-activated protein kinase or MAPK) is a serine kinase recently demonstrated to become activated by shear stress (9). Although the expression level of P38 was not affected by the flow in cultures expressing or lacking the VE-cadherin gene, P38 phosphorylation (pP38) mediated by the flow was restricted to cultures expressing the VE-cadherin gene (Fig. 6A). After the exposure of endothelial cells to physiological levels of LSS, Akt1 becomes phosphorylated (27, 28). Akt1 phosphorylation also was associated with the activation of the VEGFR2 by either VEGF-A or shear stress (27, 28). Although Akt phosphorylation was observed in VE-cad+/+ cells after their exposure to shear stress, it was not induced in the VE-cad−/− cells (Fig. 6B).
Shear stress-mediated signaling into the endothelium also affects the transcription of many endothelial genes (29, 30). This transcriptional activity is mediated by positive or negative promoter elements termed SSREs. To test the role of the complex VE-cadherin–β-catenin–VEGFR2 in shear stress-mediated transcription, arterial endothelial cells, VE-cad+/+ and VE-cad−/− cells were transfected with a construct containing the luciferase reporter gene regulated by a hybrid promoter containing the PDGF-A/SSRE (21) and exposed to shear stress. The expression of the luciferase gene was induced in both arterial endothelial cells and the VE-cad+/+ cultures in response to flow but was unaffected in the VE-cad−/− cells. In summary, the lack of VE-cadherin (and thus the lack of the VEGFR2–VE-cadherin–β-catenin complex) resulted in the abrogation of acute (activation P38 and Akt, VEGFR2 nuclear translocation) and more chronic (transcription) shear stress-mediated cellular signals.
Discussion
The present study demonstrates that after the exposure of endothelial cells to minutes of shear stress, the adherens junctions molecules VE-cadherin and β-catenin migrate to the site of cell-to-cell contacts and that a complex containing VE-cadherin, β-catenin, and VEGFR2 is formed rapidly. Shear stress also induces VEGFR2 nuclear translocation, and although the mechanism of this translocation is unknown, our findings demonstrate that it depends on the expression of the VE-cadherin gene. In endothelial cells lacking the VE-cadherin gene the activation of the serine kinases Akt1 and P38 and the induction of SSRE-dependent gene transcription do not occur. Thus, we propose that the formation of the VE-cadherin–β-catenin–VEGFR2 complex is important in shear-stress sensing and that the adherens junctions serve, among other structures, as a shear-stress transducer.
The definition of a shear-stress receptor is important in our attempts to understand the role of shear stress in various physiological and pathological conditions in the vascular system and may lead to the development of new diagnostic and therapeutic strategies. In recent years several molecules on the endothelial cell surface have been suggested to act as mechanical receptors. Some of these structures evoke identical shear stress-mediated signals such as the activation of Erk1/2, which is activated rapidly by shear stress, activation that was shown to depend on the integrins αvβ3 and α5β1 (9, 31–33) and caveolin-1 (34). Therefore, the orchestration of the response of the various shear-stress receptors is important in determining which intracellular signals will be induced in the cells. VEGFR2, similar to several other tyrosine kinase receptors, undergoes tyrosine transphosphorylation after its activation and is capable of creating complexes with various cytoplasmic and membranal proteins (35, 36). VEGFR2 can bind to the adherens junctions and several integrin pairs and affect NO production via endothelial NOS, localized in the caveolae (35). We postulate that VEGFR2, which is activated rapidly by shear stress in vascular endothelial cells, may serve as a “mediator” molecule, enabling the cross-talk between various shear-stress receptors, among them integrins, caveolae, or other tyrosine kinase receptors. This ability of VEGFR2 to form complexes with more than one surface structure also may explain the versatility in the cellular responses to physiological or pathological shear stresses.
The ability of shear stress to activate VEGFR2 and induce the formation of the VE-cadherin–β-catenin–VEGFR2 complex raises the question of whether additional tyrosine kinase receptors can serve as mechanical receptors. Recently it was shown that exposure of smooth muscle cells to short intervals of cyclic strain lead to the activation of the PDGF receptor α (37). The fact that biomechanical forces are capable of mimicking biochemical signaling by using known tyrosine kinase receptors as mechanical receptors opens a new window to our understanding mechanical signaling in various tissues. This understanding may mark the development of novel vascular therapeutic targets.
One of the rapid responses of VEGFR2 to shear stress was its migration into the nucleus. Neither the role of the nuclear VEGFR2 nor the mechanism of its translocation is understood at present. Nuclear translocation of additional tyrosine kinase receptors such as fibroblast growth factor and epidermal growth factor also was demonstrated and shown to be accompanied by transcriptional activity of the receptors (24, 25). Migration of fibroblast growth factor receptor into the nucleus is stimulated by the binding of fibroblast growth factor to the receptor, mediated by importin 2, and correlates with increased expression of cyclin D1 (38). In the present study we demonstrated that VEGFR2 nuclear translocation depended on the expression of VE-cadherin. The potential role of the complex VEGFR2–VE-cadherin–β-catenin in VEGFR2 nuclear translocation calls for further investigation.
Arteriogenesis naturally occurs in the heart and peripheral organs in rare examples of patients suffering from ischemic diseases (39). Several groups have pointed to shear-stress changes as a primary stimulus for arteriogenesis (7, 8, 10, 39) and to the involvement of MCP-1 and TGF-β in this process (40). Both vasculogenesis and angiogenesis are highly dependent on VEGF levels and are initiated by the binding of VEGF-A to its receptor VEGFR2, resulting in the formation of the VE-cadherin–β-catenin–VEGFR2 complex and the activation of the survival and proliferation signals (15, 26). Nevertheless, as shown recently by Deindl et al. (39) arteriogenesis cannot be initiated or further augmented by VEGF administration. In the present study we have shown that shear stress can mimic VEGF-A by rapidly inducing the formation of the VE-cadherin–β-catenin–VEGFR2 complex and activation of cellular signaling cascades. Yet, although similar initial events occur in response to VEGF or shear stress treatment, the intracellular signals promoted by these stimuli diverge. For example, nuclear translocation of VEGFR2 in response to VEGF-A treatment occurs hours after the treatment, whereas shear stress mediates the process within minutes. We hypothesize that shear stress by mimicking VEGF-A triggers the initial steps of arteriogenesis and eliminates the need for VEGF-A in this process. The ability of shear stress to induce the expression of MCP-1 and TGF-β rapidly as well as diverging cellular signals induced by shear stress and VEGF-A may explain the differences between VEGF-A- and shear stress-mediated blood-vessel formation.
Acknowledgments
This work was supported in part by Israel–United States Binational Scientific Fund (BSF) Grant 1998114 (to N.R.).
Abbreviations
- VE
vascular endothelial
- VEGF
VE growth factor
- VEGFR
VEGF receptor
- BAEC
bovine aortic endothelial cell
- PDGF
platelet-derived growth factor
- SSRE
shear stress response element
- LSS
laminar shear stress
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gimbrone M A, Jr, Topper J N, Nagel T, Anderson K R, Garcia-Cardena G. Ann NY Acad Sci. 2000;902:230–240. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 2.Malek A M, Alper S L, Izumo S. J Am Med Assoc. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 3.Davies P F. J Vasc Res. 1997;34:208–211. doi: 10.1159/000159224. [DOI] [PubMed] [Google Scholar]
- 4.Resnick N, Yahav H, Schubert S, Wolfovitz E, Shay A. Curr Opin Lipidol. 2000;11:167–177. doi: 10.1097/00041433-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Langille B L, O'Donnell F. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 6.Langille B L, Reidy M A, Kline R L. Arteriosclerosis. 1986;6:146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- 7.Egginton S, Zhou A L, Brown M D, Hudlicka O. Cardiovasc Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 8.Van Royen N, Piek J J, Schaper W, Bode C, Buschmann I. J Nucl Cardiol. 2001;8:687–693. doi: 10.1067/mnc.2001.118924. [DOI] [PubMed] [Google Scholar]
- 9.Shyy J Y. Biorheology. 2001;38:109–117. [PubMed] [Google Scholar]
- 10.Carmeliet P. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Collen D. J Pathol. 2000;190:387–405. doi: 10.1002/(SICI)1096-9896(200002)190:3<387::AID-PATH595>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Chen K D, Li Y S, Kim M, Li S, Yuan S, Chien S, Shyy J Y. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara K, Masuda M, Osawa M, Kano Y, Katoh K. Cell Struct Funct. 2001;26:11–17. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- 14.Dejana E, Bazzoni G, Lampugnani M G. Exp Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P, Lampugnani M G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, et al. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 16.Esser S, Lampugnani M G, Corada M, Dejana E, Risau W. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 17.Noria S, Cowan D B, Gotlieb A I, Langille B L. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 18.Schnittler H J, Puschel B, Drenckhahn D. Am J Physiol. 1997;273:H2396–H2405. doi: 10.1152/ajpheart.1997.273.5.H2396. [DOI] [PubMed] [Google Scholar]
- 19.Remuzzi A, Dewey C F, Jr, Davies P F, Gimbrone M A., Jr Biorheology. 1984;21:617–630. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- 20.Gimbrone M A, Jr, Cotran R S, Folkman J. J Cell Biol. 1974;60:673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khachigian L M, Anderson K R, Halnon N J, Gimbrone M A, Jr, Resnick N, Collins T. Arterioscler Thromb Vasc Biol. 1997;17:2280–2286. doi: 10.1161/01.atv.17.10.2280. [DOI] [PubMed] [Google Scholar]
- 22.Resnick N, Collins T, Atkinson W, Bonthron D T, Dewey C F, Jr, Gimbrone M A., Jr Proc Natl Acad Sci USA. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Venema V J, Venema R C, Tsai N, Caldwell R B. Biochem Biophys Res Commun. 1999;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- 24.Maher P A. J Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S Y, Makino K, Xia W, Matin A, Wen Y, Kwong K Y, Bourguignon L, Hung M C. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P, Collen D. Ann NY Acad Sci. 2000;902:249–264. doi: 10.1111/j.1749-6632.2000.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher A M. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 28.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Acta Physiol Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen B P, Li Y S, Zhao Y, Chen K D, Li S, Lao J, Yuan S, Shyy J Y, Chien S. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Cardena G, Comander J, Anderson K R, Blackman B R, Gimbrone M A., Jr Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traub O, Monia B P, Dean N M, Berk B C. J Biol Chem. 1997;272:31251–31257. doi: 10.1074/jbc.272.50.31251. [DOI] [PubMed] [Google Scholar]
- 32.Traub O, Berk B C. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 33.Ishida T, Peterson T E, Kovach N L, Berk B C. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 34.Park H, Go Y M, Darji R, Choi J W, Lisanti M P, Maland M C, Jo H. Am J Physiol. 2000;278:H1285–H1293. doi: 10.1152/ajpheart.2000.278.4.H1285. [DOI] [PubMed] [Google Scholar]
- 35.Bussolino F, Serini G, Mitola S, Bazzoni G, Dejana E. EMBO Rep. 2001;2:763–767. doi: 10.1093/embo-reports/kve181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter T. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Bock G, Wick G, Xu Q. FASEB J. 1998;12:1135–1142. doi: 10.1096/fasebj.12.12.1135. [DOI] [PubMed] [Google Scholar]
- 38.Reilly J F, Maher P A. J Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deindl E, Buschmann I, Hoefer I E, Podzuweit T, Boengler K, Vogel S, van Royen N, Fernandez B, Schaper W. Circ Res. 2001;89:779–786. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- 40.van Royen N, Hoefer I, Buschmann I, Heil M, Kostin S, Deindl E, Vogel S, Korff T, Augustin H, Bode C, Piek J J, Schaper W. FASEB J. 2002;16:432–434. doi: 10.1096/fj.01-0563fje. [DOI] [PubMed] [Google Scholar]