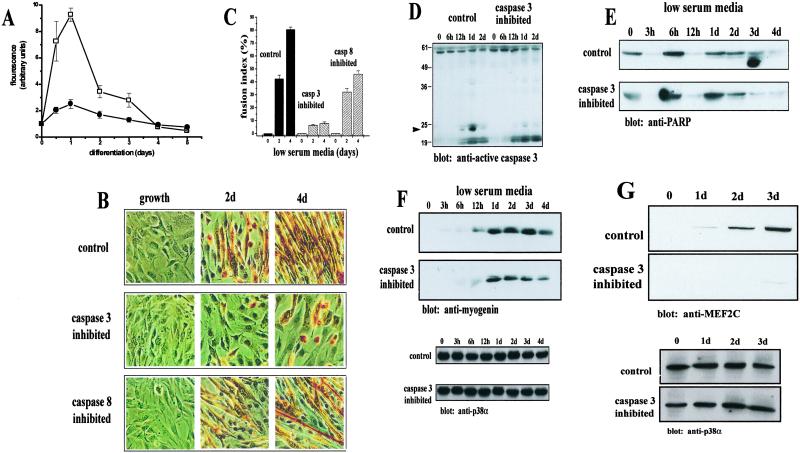
Fig 2.
Caspase 3 activity is required for skeletal muscle differentiation. (A) A sharp increase in caspase 3 activity (□) was found in C2C12 myoblasts after 1 day of low-serum treatment. A much smaller increase in caspase 8 activity (•) was noted, although a similar trend in activation was found (mean ± SEM., n = 5). (B) Morphology of C2C12 cells after inhibition of caspase 3 or caspase 8 activity. Cells were incubated in low-serum media with inhibitors specific for either caspase 3 (Z-DEVD.fmk) or caspase 8 (Z-LETD.fmk) or DMSO (control) and stained with MF20. Myotube formation is drastically attenuated in caspase 3-inhibited cells after a 4-day treatment. A minor attenuation of myotube formation is observed with caspase 8 inhibition. (C) Caspase 3-inhibited cells lack fusion capacity. Calculation of myoblast fusion indices as a percentage of cells containing two or more nuclei within a differentiated myotube (MF20 positive cell). Values were determined as mean ± SEM from three to four independently derived cultures. (D) Western blot analysis of active caspase 3 from control and caspase 3-inhibited cells. The arrow indicates a 21-kDa caspase 3 fragment, indicative of caspase 3 activation abundant in control cells and at very low levels in caspase 3-inhibited samples. (E) Comparable levels of PARP cleavage in both control and caspase 3-inhibited cells. PARP cleavage was accessed by Western blot. Shown is the 118-kDa PARP species. (F) Accumulation of myogenin is delayed and is in lower abundance in caspase 3-inhibited C2C12 myoblasts. The level of myogenin in control cells is approximately twice that of in caspase 3-inhibited cells by day 4. Equal loading was accessed by Western blot with anti-p38α (shown below). (G) Caspase 3-inhibited C2C12 myoblasts express very low levels of MEF2C relative to control cells. Equal loading (shown below) was accessed as in F. Band intensity was assessed by using SCION IMAGE.