Abstract
In this study, we have unveiled an SPR biosensor for highly sensitive and rapid detection of cancerous cells employing 2D materials. The proposed sensor structure introduces a novel approach to cancer cell detection, offering a new perspective in biosensing technology. In other words, specifically, ZnO, Si3N4 and TMDCs based plasmonic sensor structures demonstrate great potential whenever high-accuracy detection of cancerous cells is required. We present four different configuration of SPR based sensor designed for the detection of different types of cancer cells. The designed conventional configuration consists of four functional layers namely Ag, ZnO, Si3N4 and sensing medium along with BK7 prism for coupling light at the interface of metal-dielectric. To illustrate performance improvement, enhancement of light absorption capacity and achieve higher sensitivity of sensor, we incorporated four different 2D materials, MoS2, MoSe2, WS2 and WSe2. Using the angular interrogation method, the proposed layered structure, BK7/ZnO/Ag/Si3N4/WS2/sensing medium, demonstrated the highest overall sensitivity in terms of detecting three cancerous cells, blood cancer (Jurkat), cervical cancer (HeLa) and skin cancer (Basal) from healthy cells. Notably, the sensitivity which is achieved with the configuration BK7/ZnO/Ag/Si3N4/WS2/sensing medium for blood cancer detection from healthy cells with the value of 342.14 deg/RIU and FOM equal to 124.86 RIU−1, outperforms all other proposed configurations. Applying finite element method (FEM) simulations, the distribution of electric field across the interfaces of the SPR sensor configurations are analyzed. Overall, SPR biosensors offer a promising technology for the early and accurate cancerous cell detection. The high sensitivity, specificity, and ability to analyze complex biological samples make them valuable tools in the struggle against cancer. However, further research and development are demanded to optimize the performance and translation of these sensors into clinical and real-world applications.
Keywords: SPR, Biosensor, Cancer detection, TMDCs, ZnO, Sensitivity
Subject terms: Computational biophysics, Two-dimensional materials, Imaging and sensing, Nanophotonics and plasmonics, Biosensors
Introduction
According to the declaration of the World Health Organization, one of the main causes of death around the world is cancer. Significant efforts have been made to develope early, accurate and cost-effective methods and treatments. The various types of cancer can be detected early using different methods and techniques such as peptide-based optical biosensors, electroactive material-based immunosensors, plasmonic fiber optic biosensors, fluorescence spectroscopy, surface plasmon resonance (SPR) biosensor, Colorimetric biosensors and etc1–5. Notwithstanding notable advances in diagnosis of cancer, researchers intend to recognize accurate treatment methods and effective screening with a focus on the identification and application of disease biomarkers6.
The surface plasmon resonance (SPR) is a widely used technique for the on-the-spot detection of biomarkers in cancers since it can evaluate biomarkers with outstanding selectivity, rapid and reproducibility and can identify different types of cancers. Furthermore, SPR biosensors are used for studying molecular binding, molecular interactions and investigating the changes of conformation of biomolecules and ions for application in various fields such as environmental sciences, medicine, engineering and etc. The sensing mechanism of SPR biosensor is based on the change in the refractive index (RI) of the sensing medium for determination of molecule binding at a metal surface. By incorporating various layers and combinations, researchers have tried to improve the sensitivity of the SPR biosensors which paves the way for detecting subtle changes in the sensing layer for diseases diagnosis6–9. Different designs and combinations of nanomaterials, thin films and two-dimensional (2D) materials such as carbon, gold and silver nanomaterials, graphene and transition-metal dichalcogenides (TMD or TMDCs) have been proposed for high-sensitivity plasmonic biosensors10,11. Different configurations of SPR biosensor focus on the sensitivity enhancement and detection of small biomolecules and multiple analytes simultaneously. Moreover, SPR biosensor is usable for resolving the shortcomings of some old approaches and many studies have been done to improve sensitivity, detection limit and detection specificity of SPR technology in diagnosis of various cancers. In order to improve the SPR biosensor capabilities, some advances have been made. In a study12, a dual-chaneel SPR sensor has been designed for simultaneous detection of free/total prostate antigen (PSA) ratio in serum through an asynchronous competitive inhibition immunoassay with fPSA@Au nanoparticles. The results exhibited significant increase in sensitivity of free PSA with a linear correlation from 0.010 to 0.40 ng/ml. The suggested method was effective and usable for recognizing prostate cancer from benign prostatic hyperplasia. Since PSA is recognized among primary biomarkers in order to monitor prostate cancer for clinical use, so when its level is above 4ng/ml, it may indicate the presence of prostate cancer. Furthermore, it has been suggested that for investigating the biospecific and analyte-ligand interactions, the SPR is one of the best choices13,14. In a study, Mahani et al.15 employed molecular dynamics simulations to quantified low concentration of PSA protein in serum samples. They proposed an LSPR biosensor by conjugating Au nanoparticles with anti-PSA with a calibration sensitivity of 43.75 nm/(ng/mL) for the early-stage prostate cancer detection. Additionally, a numerical study of SPR sensor for early infectious illness diagnosis and pathogen detection using a multi-layered structure of silicon and selenium was conducted with a maximum sensitivity of 147.68 deg/RIU16. As well as, employing the same numerical analysis, for the rapid detection of SARS-COV-2 and Infuenza-A virus, an SPR sensor of two different arrangement consisting of gold, PtSe2, Graphene and gold, BaTiO3 and Graphene have been designed. It has exhibited notable enhancement of selectivity and sensitivity for viral samples identification17. Considering the dependency of SPR performance on the configuration parameters, an SPR biosensor using PtSe2 and BluePWS2 was designed and simulated using COMSOL multiphysics software. The maximum sensitivity of 234 deg/RIU and detection accuracy of 7.8 deg−1 illustrate its capability of the proposed to accurately and rapidly detect target analytes18.
The paramount importance of access to clean and safe drinking water is undeniable, otherwise, polluted and contaminated water may lead to many diseases, affect body tissues and produce public health concerns. Furthermore, SPR sensors, as a powerful analytical tool, have been used for detecting a wide range of biosamples including viruses, bacteria, hazardous ions, environmental pollutants, pathogens and others which have contributed to water pollution over the years. Besides, utilization of SPR biosensor for sensing of bio and chemical samples in drinking water have attracted great attention. In the 21 st century, the detection of heavy metal ions in water and accessing to clean water has one of the most challenging global issue. Accordingly, SPR sensor demonstrated its strong capacity in water environmental monitoring and for improving the water treatment efficiency to reduce energy consumption, as well. Implementing nickel, Al2O3, blue phosphorous and WSe2 in an optical surface plasmon resonance device (OSPRD) was developed to the common detector for chemical and biomedical applications with sensitivity equal to 298.55 deg/RIU19. Additional, revealing pathogens including Escherichia coli, Shigella flexneri, Vibrio cholera, and Salmonella flagellin in drinking water is important. M.G. Daher et al. employing layers of BiFeO3 and black phosphorus in an SPR sensor proposed an effective sensing platform for detection of the mentioned pathogens with outstanding sensitivity20. Recently an SPR bio-photonic sensor is developed for the quick detection of a hazardous bacteria named Vibrio cholera employing BaF2 prism, Ag, Silicon and Graphene. The bio-photonic sensor showed specific sensitivity and selectivity compared with other studies, and had a sensitivity of 307.81 deg/RIU. The improved sensitivity was the result of increasing the thickness of Ag and Si layers21.
In the same manner detecting certain genetic alternations like mutations in the BRCA1 and BRCA2 genes, holds significant importance in proactive screening for breast and ovarian cancers22. In the hope of early breast cancer diagnosis, an SPR biosensor with high sensitivity and a detection limit below 50 nM for related mismatch sequence detection is proposed by Carrascosa et al.23. A computational method was employed to develop a fiber optic SPR sensor for BRCA-1 and BRCA-2 genes mutations detection where the sensor surface was coated with graphene. The increment of sensitivity of the device confirmed the effectiveness of the proposed structure due to the absorption ability of graphene24. In a study by Uniyal and colleagues, instead of graphene, a composite structure consisting of carbon nanotubes (CNTs), MXene and TiSi2 was used in an SPR sensor for haemoglobin detection. They reported improved sensor performance, with sensitivity increasing to 300 degree/RIU25. Employing silver and zinc selenide in a sensitive SPR sensor for the detection of the chikungunya virus in the blood sample, the geometry of each layer was optimized to enhance the overall performance. According to the simulation results, the proposed structure has significantly improved the sensor performance26. For dengue virus detection through dengue-infected blood cells, a hybrid Au/BaTiO3/WSe2-based SPR biosensor was designed. The specific features of BaTiO3 such as high dielectric and refractive index and also low dielectric loss makes it suitable material for improving the performance of sensor. Analysis of the output of the proposed structure showed that the sensitivity was higher than the conventional sensors27.
Moreover, an innovative modified SPR biosensor incorporating gold and zinc oxide nanocomposite has been developed for the detection of Carbohydrate Antigen 15.3 (CA15-3), a tumor marker for breast cancer. The sensitivity has been improved and detection limit was 0.025 U/mL28. In the hope of early diagnosis of malignant tumor, in 2015, a research team measured CA15-3 tumor marker and copper concentrations in breast cancer serum using an SPR sensor based on gold nanorods. The method yielded a high sensitivity which proved to be an effective tool for breast cancer detection29. In addition to CA15-3, other biomarkers such as HER2 proteins can also be detected using SPR biosensors to enable earlier diagnosis of breast cancer. An optical fiber-based SPR optrodes coated with gold arranged in a sandwich configuration is utilized by Loyez et al. to increase the sensitivity to HER2 proteins30. For the detection of colon cancer, Springer et al. proposed an SPR biosensor utilizing gold nanoparticles to quantify carcinoembryonic antigen (CEA) in human blood plasma whose sensitivity has been improved for clinical applications31. Moreover, compared to other detection methods, SPR sensor can be used to determine organic combinations in urine of human bodies and also urea level in blood which are important for diagnosis of heart and kidney illnesses. A surface plasmon resonance–based biosensor (SPRBB) employing Ag and Si layers is suggested by32 with the sensitivity of 373 deg/RIU. It could directly determine and analyze the various levels of urea in blood.
Besides, some spectroscopy-based biosensors with high sensitivity have been designed for cancer-affected cells in various part of human body identification at early stage and in short time for better treatment. Examples of some cancerous cells are reported as blood cancer (Jurkat), skin cancer (basal), phaeochromocytoma adrenal glands cancer (PC12), cervical cancer (HeLa), breast cancer-type 1 (MDA-MB-231), breast cancer-type 2 (MCF-7)33–36. With several advantages, the SPR sensors can potentially be applied to detect cancerous cells at early stages and provides an analysis method which is real-time, label-free, rapid and sensitive. Uniyal et al.37 put forward a novel SPR sensor for biomolecules and cancerous cells early detection with outstanding sensitivity of 263.57 deg/RIU and detection accuracy equal to 0.207 deg−1 for Breast-II cancer. The structure was based on InP and Ti3C2Tx MXene materials.
Totally, for the detection of cancerous cells, the refractive index of both normal cell and cancerous cell is compared, thus the variation of some important optical parameters can be measured. In a different architecture, Sharma et al. suggested a two-dimensional photonic crystal senor with a grating design which demonstrated enhanced sensitivity for detecting cervical, basal and breast cancer cells38. Using finite element method, a novel bowl-shaped mono-core titanium coated SPR sensor was designed for the rapid detection of aforementioned cancerous cells. The sensor exhibits high wavelength sensitivity and birefringence with a resolution of the sensor varies between 1.5 × 10−2 and 9.33 × 10−3 RIU39. Moreover, identification of a cancer cell using spectroscopic optical sensor was also studied by Parvin and his colleagues. They introduced a novel design based on compact cladding monomode PCF with silica as a dielectric material. The designed structure achieved enhanced sensitivity and low confinement loss, allowing for the effective detection of blood cancer, breast cancer, skin cancer, cervical cancer and adrenal cancer cells with high sensitivity under X-polarization mode40. One of the significant improvements was shown in a SPR biosensor reported by Mostufa et al., who developed a multilayer-coated SPR sensor incorporating hybrid TiO2/Au/graphene layers. This innovative sensing device exhibited significantly augmented angular sensitivity for six different types of cancer detection such as breast (MCF-7) cancer cells and others. Additionally, the detection accuracy (DA), figure of merits (FOM) and the distribution of the electric and magnetic field were evaluated41. Considering the working principle of SPR sensors, a simple, cost effective of Ag-based SPR biosensor incorporating a new semiconductor material- zinc selenide (ZnSe)- was proposed by42. This design aimed at improving sensitivity for biomedical applications.For identification of cancer cell from normal cell and also for glucose concentration detection, the biosensor revealed high sensitivity of 359 deg/RIU and 366.6 deg/RIU, respectively.
Karki et al. proposed a two-dimensional heterostructure composed of TiSi2 and black phosphorus for the rapid detection of various cancerous cells. The results presented a relatively improved sensitivity compared to earlier designs43. Considering the fact that normal and cancerous cells differ in their refractive indices, an SPR biosensor combining graphene oxide (GO) and Si3N4 was developed to detect different types of cancer cells. The biosensor presented better performance in comparison to previous studies with a maximum sensitivity of 245 deg/RIU for breast cancer type 144. Aiming at early cancer detection, Kumar and colleagues designed an SPR biosensor using titanium dioxide (TiO2), and graphene layers. For the detection of adrenal gland (PC12) cells, the proposed sensor displayed high and enhanced sensitivity45.
In the present study, using a nanomaterial-based SPR biosensor, we aim to detect various cancerous cells: skin cancer (basal), blood cancer (Jurkat) and cervical cancer (HeLa). The sensor features a multilayer structure consisting of BK7 glass prism coated with layers of ZnO, Ag, Si3N4, TMDCs and sensing medium. Numerical simulations demonstrate that the sensor achieves enhanced performance in terms of sensitivity, detection accuracy (DA), figure of merit (FoM) and signal-to-noise ratio (SNR) compared to other reported designs. These improvements are observed for both healthy and cancerous cells, suggesting that the proposed configuration could serve as a promising platform for early and highly sensitive cancer diagnosis.
Theoretical method
Description of sensor’s constituent layers
In recent years, advances in nanocomposite-based biosensors have helped overcome some limitations in biosensing and the rapid detection of cancer cells. In that sense, surface plasmon resonance (SPR) method should not be ignored due to its nature strengthen level for nanosensing. Hence, we have introduced an SPR-based optical sensor aimed at investigation of three different types of cancer affected cells: blood cancer (Jurkat), cervical cancer (HeLa) and skin cancer (Basal). The approach relies on differences in the refractive index (RI) between healthy cells and cancerous cells- changes produced by the mutated genes- which provides a considerable basis for distinguishing between them. Thus, the designed structure will be a promising tool as a sensing device for identification of the chemical or ingredient modifications within the cells.
In general, achieving the desired sensitivity and specificity for biomolecule detection in SPR biosensors requires careful consideration and optimization of specific operational parameters. The first key factor lies in the selection of materials, such as the prism, metal thin film, and other layers, as well as the order of the layers and the optimization of their thickness. Additionally, effective functionalization of the surface, if needed, plays a crucial role. The material used in the biomolecular recognition layer is also of great importance, as it directly impacts the sensor’s performance. Another critical parameter involves operational conditions, such as the angle and wavelength of the incident light. The refractive index (RI) of the prism, which couples light into the metal layer, and the refractive index of the sensing medium must be carefully considered to achieve optimal results. To ensure the best performance of the proposed sensor, advanced fabrication techniques should be employed for the precise incorporation of layers and integration of nanomaterials. Proper optimization of all parameters and components will ultimately lead to label-free and real-time detection of biomolecules and cancerous cells.
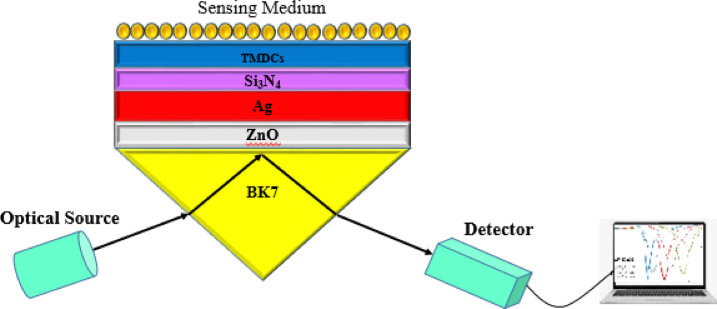
The proposed structure shown in Fig. 1 is based on Kretschmann configuration consisting of a BK7 Prism-ZnO-Ag-Si3N4-TMDCs-sensing medium.
Fig.1.
Schematic diagram of the SPR biosensor by using ZnO, Si3N4 and TMDCs for sensitivity enhancement.
In that strategy, various prisms were evaluated and according to the best performance of the sensor, the BK7 prism was selected as the dielectric substrate. An electromagnetic light source with a wavelength of 633 nm was used in the setup. The semiconductor ZnO, a good adhesion layer material, provides significant enhancement in biosensor sensitivity due to its ability to efficiently gather light and making the larger shift in resonance angle. The interface between metal and ZnO layer operates as phonon interaction. Moreover the interaction at ZnO-metal interface eventuates to band flattering46,47. Considering mentioned properties, ZnO is a promising candidate for integration into SPR biosensor structure. However, Silver (Ag) as a plasmonic material possesses excellent optical properties and relatively low oxidation resistance. These properties contribute to high detection accuracy and make Ag an appropriate choice for sensing applications. Moreover, Ag offers high electrical conductivity and also high reflectivity which are essential for supporting surface plasmon resonances and minimum energy loss during the excitation of surface plasmons48,49. Besides, it supports both propagating and localized surface plasmon modes. In addition, its moderate biocompatibility and antimicrobial properties provide a suitable platform for biosensing applications50. Nowadays, Silicon Nitride (Si3N4), as a dielectric material with a wide bandgap of ~ 5 eV has developed exceptional properties. Silicon Nitride (Si3N4), offers optical transparency in the visible to near-infrared range51 which facilitate an efficient light-matter interaction. In addition it has mechanical and chemical stability and resistance to corrosion which are important in biosensing and make it a suitable substrate material for sensors52,53. In our proposed biosensor structure, the application of Si3N4 layer in combination with the Ag layer leads to fix the oxidation issue of this material, increasing the lifetime of the sensor, and enhancing sensor performance54–56.
In the design of proposed structure, different number and thickness of TMDCs (Molybdenum diselenide (MoSe2), Molybdenum disulfide (MoS2), Tungsten diselenide (WSe2) and Tungsten disulfide (WS2)) layers across multiple models were utilized to investigate their enhancement impact on the sensitivity of SPR biosensor. Because of the need for improved sensitivity and optimum performance of biosensor, we have considered alternative layers with various thicknesses. Thus, in order to determine the optimized thickness of Ag layer, thickness values ranging from 30 to 55 nm with the interval of 5 nm were evaluated. It was noticed that a 55 nm thick Ag layer provided the best performance with the lowest reflectance angle. As mentioned, the refractive index and thickness of each layer are among crucial parameters which affect the sensitivity and performance of SPR biosensor. Therefore, to achieve the best sensing performance of biosensor, the optimized thickness of each layer was calculated and are reported in Table 1.
Table 1.
Optimized values of thickness and refractive indices of various layers at λ = 633 nm.
| Types of materials | Thickness of monolayer(nm) | Refractive index (n + ik) |
|---|---|---|
| Prism (BK7 glass) | - | 1.5151 |
| ZnO | 5 | 1.952 + 0.02i |
| Silver (Ag) | 55 | 0.056206 + 4.2776i |
| Si3N4 | 5 | 2.0394 |
|
MoS2 MoSe2 WS2 WSe2 |
0.65 0.7 0.8 0.7 |
5.08 + 1.1723i 4.62 + 1.0063i 4.9 + 0.3124i 4.55 + 0.4332i |
The different thickness of other layers were tested and compared with different literatures for better results and more accurate data. In other words, using TMDCs is one of the engaged alternatives for increasing the adsorption rate of biomolecules of sensor surface, so the sensitivity improvement. The enhancement of sensitivity is due to the dielectric constant of TMDCs. Two parts of dielectric constant, the real and imaginary parts which are responsible for light absorption and electron energy loss, respectively, can be considered among important factors to affect sensitivity57–59. To achieve the best sensing performance of biosensor, the optimized thickness of each layer was calculated and are reported in Table 1. Furthermore, the refractive index and the optimized thickness of other materials using various references is depicted in Table 1, as well.
Sensor theoretical modeling and optical performance parameters
For the proposed multilayer model of sensor, the reflectance calculations via transfer matrix method (TMM) and Fresnel’s equations is used according to the model mentioned in our previous study8 and in the references42,44,60–63. The performance of sensor is evaluated by performance factors: sensitivity (S), quality factor (Q), detection accuracy (DA) and figure of merit (FoM) which are defined as following,
The parameter sensitivity is related to the capability of the sensor for detecting slight variations in the refractive index on the surface of sensor8,44. Thus, it is described as the ratio of shift in resonance angle (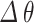 ) and the variation of RI (
) and the variation of RI ( ):
):
 |
1 |
The sensitivity is evaluated in deg/RIU. Besides, the quality factor (Q) which is known as the parameter figure of merit (FOM) in RIU−1 is defined as the ratio of reflectance sensitivity to the full-width half-maximum (FWHM) of the reflectance curve44,
 |
2 |
where the FWHM describes the width of a peak related to the amplitude of a graph at half of its maximum value and FOM explains about the resolution of SPR sensor power.
The other performance parameter of biosensor is detection accuracy (DA). This parameter illustrates about the effectiveness and the quality of the sensor and can be defined as the following relation
 |
3 |
From SPR characteristic curves, the parameter signal-to-noise ratio (SNR) of the real SPR sensing system can be written as given,
 |
4 |
This sensing parameter defines that using real instruments, how well the signals can be measured41,44,64.
Practical fabrication process
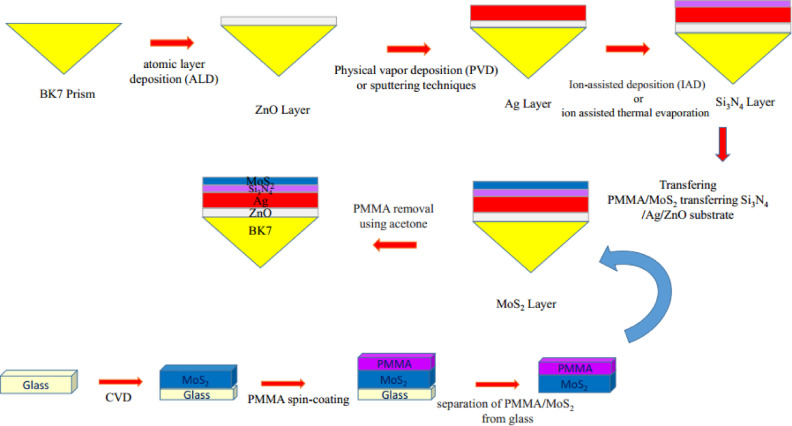
In this paper, we theoretically investigated and proposed a hybrid structure BK7/ZnO/Ag/Si3N4/TMDCs/sensing medium-based biosensor using numerical modelling. Although, the design is currently theoretical, the proposed sensor layout could be practically fabricated. According to some researches and literature findings, various methods have been applied for synthesizing ZnO, Si3N4, TMDCs and fabrication of biosensors based on these materials. In General, two main approaches- top-down and bottom-up- are commonly used for synthesizing and fabrication. The top-down approach includes techniques such as liquid, mechanical, surface functionalization, lithography, chemical exfoliation techniques and the bottom-up approach consists of chemical vapor deposition (CVD), molecular layer deposition, chemical synthesis, sol–gel synthesis and solvothermal procedures65. Zinc oxide (ZnO), a transparent semiconductor, has gained significant attention because of its unique electronic and optical properties. It also can be used as an effective adhesion layer. The ZnO layer can be deposited onto a BK7 prism using either atomic layer deposition (ALD) or thermal coating techniques, both used in thin film applications. This material can make larger shift in resonance angle so leads to sensitivity enhancement and increase in detection accuracy66. In the next step, Ag layer can be deposited using high-frequency magnetron sputtering technique or Physical vapour deposition (PVD). According to54 silicon nitride (SiNx) and Si3N4 thin films have been successfully prepared by ion-assisted deposition (IAD) or ion assisted thermal evaporation, yielding favourable optical constants. The Si3N4 layer functions as an anti-reflection coating on Ag layer, overcome the oxidation problem of Ag. This makes silver layer highly resistive to the oxidation and environmental degradation54,55,67. Each TMDCs layer, MoSe₂, MoS₂, WSe₂, and WS₂, can be deposited via the Chemical vapour deposition (CVD) technique, mechanical or chemical exfoliation, or hydrothermal/solvothermal processes68–71. Elias and his colleagues using a controlled thermal reduction–sulfurization method synthesized heterogeneous systems of MoS2 and WS2 layers with thicknesses ranging from monolayers to a few layers72. However, considering the above mentioned methods, it is reasonable to suggest that the proposed sensor layout could be fabricated in practice. The possible fabrication process which contains some steps can be explained with the help of Figs. 1 and 2 in the manuscript. At first, in order to conduct this experiment, a monochromatic light source operating a wavelength of 633 nm is typically employed. In the suggested setup, SPs are consequently excited by p-polarized light.
Fig. 2.
The potential fabrication stages for the suggested design.
According to the ATR principle, the proposed setup consists of a prism through which incident light enters on one side and exits on the other. The output component usually consists of a detector and a software-loaded system that determines and calculates the results. The potential fabrication steps of the proposed biosensor can be explained as illustrated in Fig. 2.
Results and discussion
In the present study, four different prism-based designs of an SPR sensor, labled structure I, structure II, structure III and structure IV, were simulated for the detection of different cancer cells. Spectral analysis was conducted for three specific cancer cells: blood cancer (Jurkat), cervical cancer (HeLa) and skin cancer (Basal). The first layers in our proposed sensor, in all designs are common and consisting of ZnO, Ag and Si3N4 with each layer optimized for thickness.
Considering the importance of selection of prism material and its effect on the performance of SPR sensor, we examined several prism materials and the related SPR responses were calculated. As a result, BK7 prism was selected due to its suitable resonance angle, FWHM and sensitivity. In Table 2, the result of our sensitivity calculations for some prism materials for comparison are presented. As it is obvious, the best prism material choice for our proposed configuration, is BK7 due to its appropriate refractive index in the working wavelength.
Table 2.
Analysis of Prism material effect on the sensitivity of the proposed conventional structure (Prism/ZnO/Ag/Si3N4/sensing medium) and Prism/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) for Jurkat cell detection.
| SPR sensor structure | Prism material | Sensitivity(deg/RIU) |
|---|---|---|
| Prism/ZnO/Ag/Si3N4/sensing medium | SF10 | 82.14 |
| SF11 | 67.14 | |
| 2S2G | 35.71 | |
| BK7 | 227.8 | |
| Prism/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) | SF10 | 83.57 |
| SF11 | 75.71 | |
| 2S2G | 40 | |
| BK7 | 342.14 |
In order to verify the influence of the thickness of silver layer on the performance of SPR sensor, the variation Ag layer thickness of 45 nm, 50 nm and 55 nm within the conventional structure (BK7/ZnO/Ag/Si3N4/sensing medium) and BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), and their sensitivity for Jurkat cell detection are presented in Table 3. Considering the numerical analysis of these results, it can be concluded that the sensitivity of 55 nm Ag is higher for cancerous Jurkat cells, so the optimized thickness of 55 nm was chosen for implementation in proposed SPR sensor configuration. Besides the error tolerance of the proposed sensor can be evaluated considering Table 3.
Table 3.
Optimization of Ag layer thickness.
| Structure | Silver (Ag) layer thickness (nm) | Sensitivity (deg/RIU) |
|---|---|---|
| BK7/ZnO/Ag/Si3N4/sensing medium | 45 | 211.4 |
| 50 | 214.2 | |
| 55 | 227.8 | |
| BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) | 45 | 312 |
| 50 | 320 | |
| 55 | 342.14 |
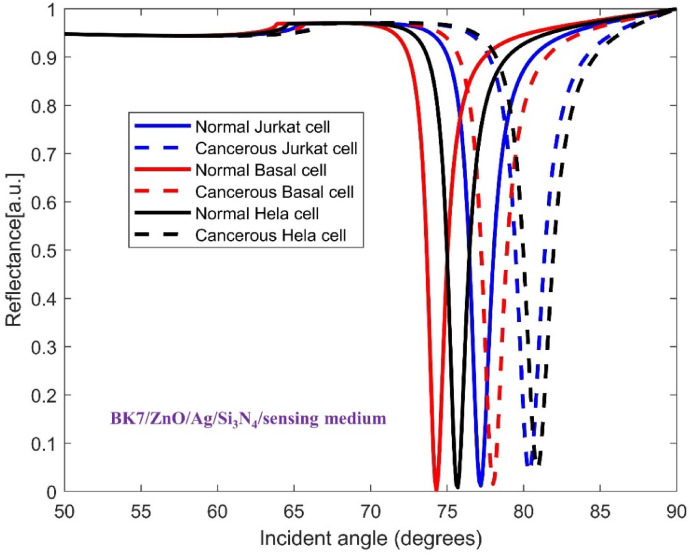
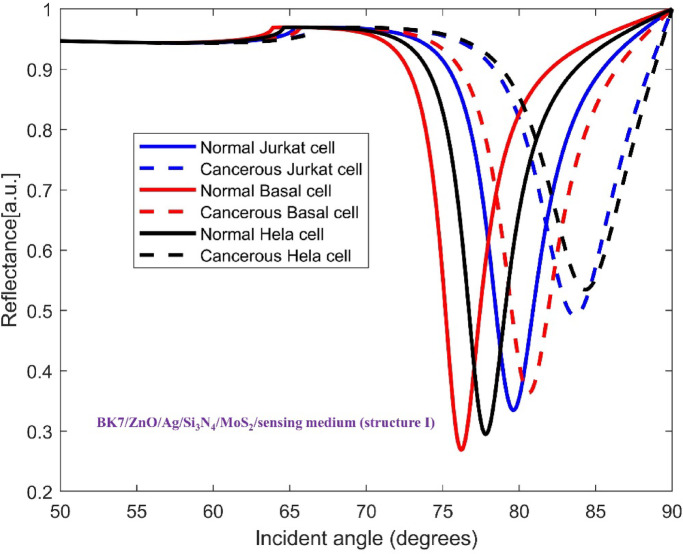
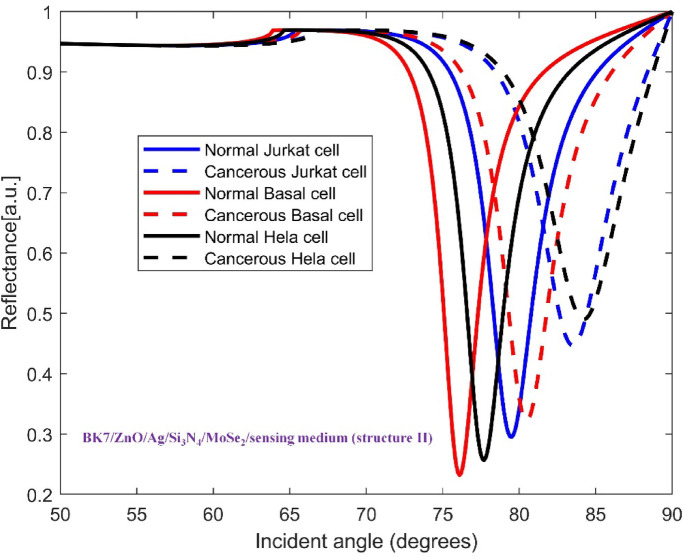
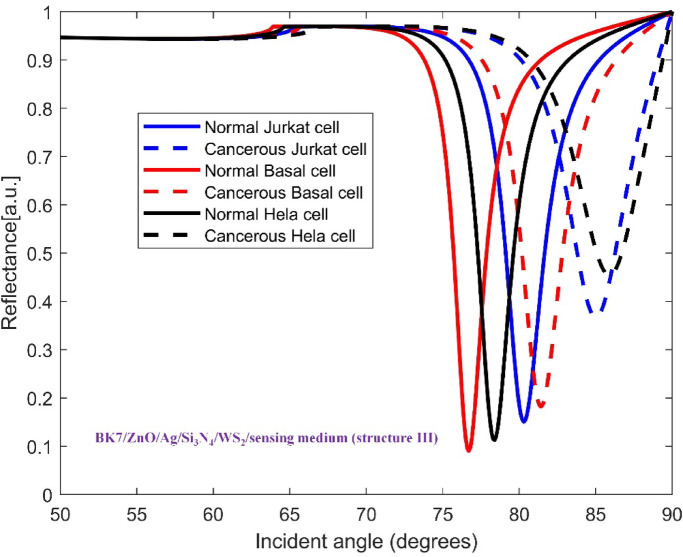
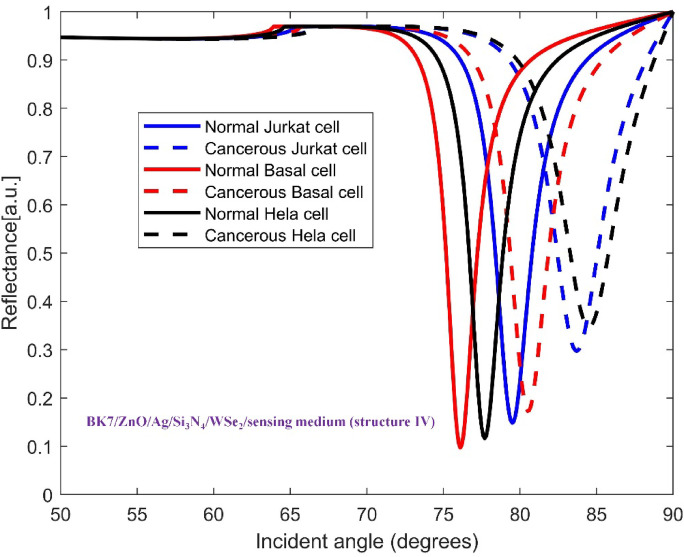
The TMDCs materials were deposited on top of the mentioned base layers to improve the sensitivity in each configuration. Hence, the architecture of SPR sensor for detecting all three cancer cell types consists of a coupling prism BK7, ZnO, Ag metal, Si3N4, one of four distinct TMDC materials and sensing medium. This layered structure allows us to analyze how each material affects the sensitivity of sensor for recognizing the normal and cancer cells. In Figs. 3, 4, 5, 6 and 7, the variation of reflectance as a function of incident light angle for both normal cells and three selected cancer cells, Jurkat cell, Basal cell and HeLa cell using conventional setup as well as the four various structures are presented and compared.
Fig. 3.
Variation of reflectance as a function of incident light angle for normal and Jurkat cell, normal and cancerous Basal cell, normal and cancerous HeLa cell detection using conventional structure (BK7/ZnO/Ag/Si3N4/sensing medium). The solid and dashed reflectance curves correspond to the normal and cancerous cells, respectively.
Fig. 4.
Variation of reflectance as a function of incident light angle for normal and Jurkat cell, normal and cancerous Basal cell, normal and cancerous HeLa cell detection using conventional structure (BK7/ZnO/Ag/Si3N4/MoS2/sensing medium). The solid and dashed reflectance curves correspond to the normal and cancerous cells, respectively.
Fig. 5.
Variation of reflectance as a function of incident light angle for normal and Jurkat cell, normal and cancerous Basal cell, normal and cancerous HeLa cell detection using conventional structure (BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium). The solid and dashed reflectance curves correspond to the normal and cancerous cells, respectively.
Fig.6.
Variation of reflectance as a function of incident light angle for normal and Jurkat cell, normal and cancerous Basal cell, normal and cancerous HeLa cell detection using (BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III)). The solid and dashed reflectance curves correspond to the normal and cancerous cells, respectively.
Fig.7.
Variation of reflectance as a function of incident light angle for normal and Jurkat cell, normal and cancerous Basal cell, normal and cancerous HeLa cell detection using (BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV)). The solid and dashed reflectance curves correspond to the normal and cancerous cells, respectively.
Result analysis of SPR biosensor for Jurkat cells (blood cancer) detection
In this section, we analyze the performance and sensitivity of SPR biosensor designed for the detection of Jurkat cells which are considered as the principal candidate in blood cancer. Detecting variations in the refractive index of blood samples of patients is simpler and more feasible than measuring the physical dimensions of cancerous cells since they are typically on the nanometer scale. Thus, SPR biosensing emerges as an effective optical technology to detect life-threatening cancers accurately. The conventional sensor structure, composed of BK7/ZnO/Ag/Si3N4/sensing medium, was evaluated alongside four modified designs of sensor with the presence of TMDCs for investigating the most efficient design. These structures are comprising BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II), BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV). Inasmuch the refractive index of normal cells increases when they become cancerous, this change can be utilized to recognize between normal and cancerous cells effectively. Figures 3, 4, 5, 6 and 7 presents the characteristic curves for the conventional and the four modified SPR sensor configurations to detect normal and Jurkat cells. Specifically, in Fig. 3 the reflectance variation as a function of incident light angle for normal and Jurkat cell recognition for conventional configuration is displayed.
It is comprehended that the resonance dip for normal cells occurs at 77.18° whereas for Jurkat cells occurs at 80.37°. Therefore, the SPR angle shift (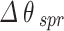 ) equals to 3.19°, and considering the difference in RI between normal and cancerous Jurkat cells, the performance of this configuration in cancerous cells detection is described by its angular shift sensitivity which is 227.8 deg/RIU. Furthermore, other sensing parameters including Figure of Merit (FOM), detection accuracy (DA) and SNR are found to be 152.88 RIU−1, 0.671 deg−1 and 2.14, respectively. For the sake of clarifying the improvement of the performance of the proposed sensor, the incorporation of the mentioned TMDCs into the conventional sensor structure have been done. As it is obvious in Fig. 4, in the presence of MoS2, i.e. forming the configuration BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), the SPR angle shift increases eventuating in sensitivity improvement in the detection of Jurkat cells which are calculated as 4.08° and 291.4 deg/RIU, respectively. In the Fig. 5, the simulation result for both normal and cancerous Jurkat cells is presented for the configuration BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II). It is evident from the graph that the sensitivity of detection of Jurkat cancer cells increases to 292.14 deg/RIU. Subsequently, to investigate the methods for improving sensitivity in cancer cells detection, WS₂ and WSe₂ layers were added to the sensor structure.
) equals to 3.19°, and considering the difference in RI between normal and cancerous Jurkat cells, the performance of this configuration in cancerous cells detection is described by its angular shift sensitivity which is 227.8 deg/RIU. Furthermore, other sensing parameters including Figure of Merit (FOM), detection accuracy (DA) and SNR are found to be 152.88 RIU−1, 0.671 deg−1 and 2.14, respectively. For the sake of clarifying the improvement of the performance of the proposed sensor, the incorporation of the mentioned TMDCs into the conventional sensor structure have been done. As it is obvious in Fig. 4, in the presence of MoS2, i.e. forming the configuration BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), the SPR angle shift increases eventuating in sensitivity improvement in the detection of Jurkat cells which are calculated as 4.08° and 291.4 deg/RIU, respectively. In the Fig. 5, the simulation result for both normal and cancerous Jurkat cells is presented for the configuration BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II). It is evident from the graph that the sensitivity of detection of Jurkat cancer cells increases to 292.14 deg/RIU. Subsequently, to investigate the methods for improving sensitivity in cancer cells detection, WS₂ and WSe₂ layers were added to the sensor structure.
To explore further enhancement in sensitivity, for the structures BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) and BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV), the reflectance vs. incident angle curves are shown in Figs. 6 and 7, respectively. Considering the unique optical and electronic properties along with strong plasmonic effects of these materials, the sensitivity of sensor increases to 342.14 deg/RIU and 303.57 deg/RIU. Moreover, the corresponding values for Figure of Merit (FOM), detection accuracy (DA) and SNR for these modified structures are presented in Table 4. The comparison between the calculated results demonstrates that the inclusion of WS2, i.e. BK7/ZnO/Ag/Si3N4/WS2/sensing medium layered structure yields the highest sensitivity for detection of cancerous Jurkat cells from normal cells among other configurations tested..
Table 4.
The performance characteristics of the various proposed SPR biosensor for Jurkat, Basal and HeLa cancerous cells concerning the normal cells.
| Structures | Cancer cells | Sensitivity (deg/RIU) | FOM, (RIU−1) | DA, deg−1 | SNR |
|---|---|---|---|---|---|
| BK7/ZnO/Ag/Si3N4 /sensing medium | Jurkat | 227.8 | 152.88 | 0.671 | 2.14 |
| Basal | 185.5 | 146.06 | 0.787 | 2.291 | |
| HeLa | 222.08 | 163.29 | 0.735 | 3.919 | |
| BK7/ZnO/Ag/Si 3 N 4 /MoS 2 /sensing medium (structure I) | Jurkat | 291.4 | 77.70 | 0.266 | 1.088 |
| Basal | 222 | 76.02 | 0.342 | 2.098 | |
| HeLa | 276.25 | 87.69 | 0.317 | 2.104 | |
| BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II), | Jurkat | 292.14 | 77.08 | 0.263 | 1.079 |
| Basal | 215.5 | 79.22 | 0.367 | 1.584 | |
| HeLa | 276.6 | 95.37 | 0.344 | 2.289 | |
| BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), | Jurkat | 342.14 | 124.86 | 0.364 | 1.748 |
| Basal | 236 | 115.12 | 0.487 | 2.302 | |
| HeLa | 315.83 | 129.97 | 0.411 | 3.119 | |
| BK7/ZnO/Ag/Si 3 N 4 /WSe 2 /sensing medium (structure IV) | Jurkat | 303.57 | 120.46 | 0.396 | 1.686 |
| Basal | 222 | 111.55 | 0.502 | 2.231 | |
| HeLa | 285 | 129.54 | 0.454 | 3.109 |
Result analysis of SPR biosensor for Basal cells (skin cancer) detection
We now turn our attention to the optimization of the sensitivity for detection of cancerous Basal cells with particular emphasis on the effect of TMDCs materials. Figure 3 displays the reflectance curves for the conventional configuration of the proposed SPR sensors for both normal and cancerous basal cells detection. Basal cells are considered one of the primary biomarkers in the skin cancer, as they originate in the outermost layer of the skin68. The RI of biomolecule solutions for healthy and cancerous Basal cells of the skin cancer are 1.36 and 1.38, respectively. As shown in Fig. 3, for conventional structure, the resonance angle shift induced by the presence of cancerous Basal cells is clearly observed. The sensitivity, Figure of Merit (FOM), detection accuracy (DA) and SNR are found to be 185.5 deg/RIU, 146.06 RIU−1, 0.787 deg−1 and 3.92, respectively.
We now turn our attention to the enhancement the sensitivity of SPR biosensor for cancerous Basal cells detection with respect to the incorporating 2D materials onto the conventional configuration. The obtained results for all structures: BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II), BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV) for both normal and cancerous Basal cells detection are summarized in Table 4. Furthermore, the Figs. 4, 5, 6 and 7 show the variation of reflectance as a function of the incident angle for the four proposed configurations with TMDCs in the detection of cancerous Basal cells. Comparing these figures, it is concluded that the influence of the presence of WS2 layer in sensing chip on the improvement of performance of the sensor is more considerable. The SPR angle shift (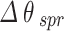 ) equals to 4.72°, resulting in an improved sensitivity of 236 deg/RIU for the BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV). As well as, the sensor performance of the four proposed structures with TMDCs for detection of healthy and cancerous Basal cells are evaluated by the measurement of sensitivity, FOM, DA and SNR for each case. As a result, for BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV), FOM is 115.12 RIU−1, DA is 0.487 deg−1, and SNR is 2.30. In summary, Table 4 provides a comprehensive comparison of the performance parameters of the proposed structures. Based on these results, the proposed biosensor shows a high capability to detect a subtle refractive index change between normal and cancerous Basal cells with significant sensitivity.
) equals to 4.72°, resulting in an improved sensitivity of 236 deg/RIU for the BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV). As well as, the sensor performance of the four proposed structures with TMDCs for detection of healthy and cancerous Basal cells are evaluated by the measurement of sensitivity, FOM, DA and SNR for each case. As a result, for BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV), FOM is 115.12 RIU−1, DA is 0.487 deg−1, and SNR is 2.30. In summary, Table 4 provides a comprehensive comparison of the performance parameters of the proposed structures. Based on these results, the proposed biosensor shows a high capability to detect a subtle refractive index change between normal and cancerous Basal cells with significant sensitivity.
Results analysis of SPR biosensor for HeLa cells (cervical cancer) detection
Nowadays, cervical cancer is one of the most commonly diagnosed cancer among women, making early and more precise detection essential for improving patient life quality. Howbeit, remarkable diagnosis methods rely on subjective assessments by physicians, highlighting the need for more standardized measuring and detection techniques of cancerous cells to support clinical decisions-making and treatments. The SPR biosensor as one of optical biosensing technologies offer a promising solution to meet these requirements. Considering the mechanism of SPR sensors which depend primarily on the changes of refractive index which result in a measurable shift in the SPR inclination, this kind of optical device is used for detection of HeLa cells, a key biomarker in cervical cancer. Normal HeLa cells become cancerous following infection with human papillomavirus 18 (HPV18). In this study, the RI of normal and cancerous HeLa cells are measured as 1.368 and 1.392, respectively and tested using the proposed biosensor configuration in the present study67,73.
The configuration of sensor for cancerous HeLa cells detection is composed of the materials and layers structure described previously. In Figs. 3, 4, 5, 6 and 7, the reflectance plots as a function of incident angle for both normal and cancerous HeLa cells using the conventional structure as well as modified configurations incorporating TMDCs are presented. The shifts in the SPR angle are influenced by the variation in the refractive index between normal cells and cancerous cells. The calculated performance parameters namely sensitivity (S), Figure of Merit (FOM), detection accuracy (DA) and SNR have been presented in Table 4. For the detection of normal and cancerous HeLa cells, the evaluated sensitivity for the proposed conventional structure and four configurations: BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II), BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV) are 222.08 deg/RIU, 276.25 deg/RIU, 276.6 deg/RIU,315.83 deg/RIU, 285 deg/RIU, respectively. The superiority of the proposed BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) sensor for cancerous HeLa cells with the highest sensitivity of 315.83 deg/RIU, Figure of Merit (FOM) of 129.97 RIU−1, detection accuracy (DA) of 0.411 deg−1 and SNR of 3.11 is evident. Furthermore, a comparative analysis of all proposed configurations is illustrated in Table 4.
It is comprehended that the resonance dip for normal cells occurs at 77.18°, while for Jurkat cells it is detected at 80.37°. Therefore, the SPR angle shift (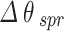 ) equals to 3.19°. Based on the refractive index difference between normal and cancerous Jurkat cells, the sensing performance of this configuration in cancerous cells detection is characterized by its angular sensitivity which is calculated to be 227.8 deg/RIU. Furthermore, other key sensing parameters including Figure of Merit (FOM), detection accuracy (DA) and SNR are found to be 152.88 RIU−1, 0.671 deg−1 and 2.14. To further clarify the improvement of the performance of the proposed sensor, the conventional sensor was modified by incorporation of the mentioned TMDCs materials into the sensing layer. As it is obvious in Fig. 4, in the presence of MoS2, i.e. with the configuration BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), the SPR anglar shift and the sensitivity of the detection of Jurkat cells are calculated as 4.08° and 291.4 deg/RIU, respectively. Similarly, in the Fig. 5, the simulation results for normal and cancerous Jurkat cells for the configuration BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II) are presented. It is evident from the plot that the sensitivity for the Jurkat cancer cells detection increases to 292.14 deg/RIU. Subsequently, to investigate the potential methods for improving sensitivity in cancer cells detection, WS₂ and WSe₂ layers were also introduced into the sensor design. Thus, for the structures BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) and BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV), the reflectance vs. incident angle graph are shown in Figs. 6 and 7. Considering the unique optical and electronic properties as wll as strong plasmonic effects of these materials, the sensitivity of sensor increases to 342.14 deg/RIU for structure III and 303.57 deg/RIU for structure IV. Moreover, the other key sensing parameters Figure of Merit (FOM), detection accuracy (DA) and SNR for these structures are presented in Table 4. The comparison between the calculated results demonstrates that the incorporation of WS2 into the proposed configuration, i.e. BK7/ZnO/Ag/Si3N4/WS2/sensing medium layered structure exhibited the highest sensitivity for detection of cancerous Jurkat cells from normal cells.
) equals to 3.19°. Based on the refractive index difference between normal and cancerous Jurkat cells, the sensing performance of this configuration in cancerous cells detection is characterized by its angular sensitivity which is calculated to be 227.8 deg/RIU. Furthermore, other key sensing parameters including Figure of Merit (FOM), detection accuracy (DA) and SNR are found to be 152.88 RIU−1, 0.671 deg−1 and 2.14. To further clarify the improvement of the performance of the proposed sensor, the conventional sensor was modified by incorporation of the mentioned TMDCs materials into the sensing layer. As it is obvious in Fig. 4, in the presence of MoS2, i.e. with the configuration BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), the SPR anglar shift and the sensitivity of the detection of Jurkat cells are calculated as 4.08° and 291.4 deg/RIU, respectively. Similarly, in the Fig. 5, the simulation results for normal and cancerous Jurkat cells for the configuration BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II) are presented. It is evident from the plot that the sensitivity for the Jurkat cancer cells detection increases to 292.14 deg/RIU. Subsequently, to investigate the potential methods for improving sensitivity in cancer cells detection, WS₂ and WSe₂ layers were also introduced into the sensor design. Thus, for the structures BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) and BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV), the reflectance vs. incident angle graph are shown in Figs. 6 and 7. Considering the unique optical and electronic properties as wll as strong plasmonic effects of these materials, the sensitivity of sensor increases to 342.14 deg/RIU for structure III and 303.57 deg/RIU for structure IV. Moreover, the other key sensing parameters Figure of Merit (FOM), detection accuracy (DA) and SNR for these structures are presented in Table 4. The comparison between the calculated results demonstrates that the incorporation of WS2 into the proposed configuration, i.e. BK7/ZnO/Ag/Si3N4/WS2/sensing medium layered structure exhibited the highest sensitivity for detection of cancerous Jurkat cells from normal cells.
Impact due to the sensor configuration on characteristics of biosensor
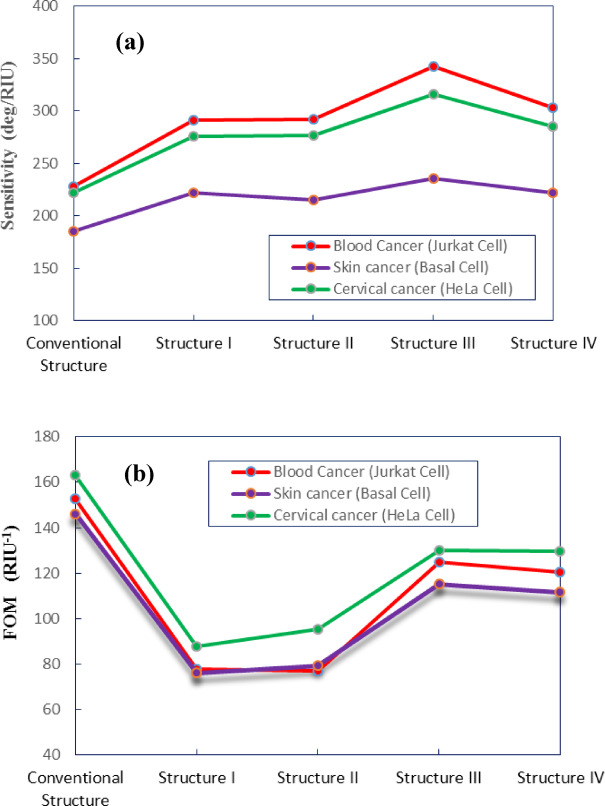
In this part, the precise performance metrics and parameters for the conventional SPR structure and the four modified configurations incorporating TMDCs are compared and depicted in Fig. 8. Advancing the analysis, in Fig. 8a the variation in sensitivity across different sensor structures for the detection of cancerous HeLa, Basal and Jurkat cells is displayed. As it is obvious in this graph for detection of cancerous cells, the combination of WS2 in structure III (BK7/ZnO/Ag/Si3N4/WS2/sensing medium) achieves the highest sensitivity among all configurations which is 342.14 deg/RIU, 236 deg/RIU and 315.83 deg/RIU for cancerous Jurkat, Basal and HeLa cells, respectively. In contrast, the conventional structure exhibits the lowest sensitivity. Comparing to other configurations, the structure IV (BK7/ZnO/Ag/Si3N4/WSe2/sensing medium) ranks second in terms of sensitivity for cancer cells detection.
Fig.8.
Performance parameters for conventional (BK7/ZnO/Ag/Si3N4/sensing medium) and BK7/ZnO/Ag/Si3N4/MoS2/sensing medium (structure I), BK7/ZnO/Ag/Si3N4/MoSe2/sensing medium (structure II), BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), BK7/ZnO/Ag/Si3N4/WSe2/sensing medium (structure IV) (a) Sensitivity (b) FOM.
It is evident that the presence of WS2 and WSe2 layers in the SPR sensor configuration, enhances the sensitivity comparing to others. Thus, due to specific optical properties such as high refractive indices, strong plasmonic properties, stability and biocompatibility, the presence of these materials can improve the performance of the sensor. Figure 8b depicts the variation in Figure of Merit (FOM) across all sensor configurations. It is comprehended that the FOM of the characteristic curve decreases from conventional structure (163.29 RIU−1) for HeLa cell detection when MoS2 and MoSe2 are introduced. Although all four structures exhibit lower FOM values than the conventional one, structures III and IV with the WS2 and WSe2 layers present better FOM in comparison to structures I and II. It can be said that the sensitivity increasing inevitably brings a decrease in quality factor and detection accuracy. The optimum sensitivity of value 342.14 deg/RIU for Jurkat cell detection and FOM equal to 124.86 RIU−1 are proposed for detection of cancerous cells using SPR sensors. Furthermore, considering other structures, the WS2 layer due to relatively small imaginary part of dielectric constant, exhibits lower energy loss compared to other TMDCs, thereby offering better improvement effect on the performance of SPR biosensor for detection of biomolecules and biochemical subastances.
Furthermore, Table 5 presents a comparative analysis of the performance of some various recently reported 2D materials-based SPR biosensors and the proposed sensor. It is obvious that the proposed structure successfully achieves the goal of improved sensing performance.
Table 5.
Comparison of sensitivity of proposed and recently investigated SPR biosensors for cancer detection.
| SPR sensor design | Type of cancer (cancerous cell) | Sensitivity (deg/RIU) | DA (deg−1) | Ref. |
|---|---|---|---|---|
| Prism/Ag/Si3N4/GO/sensing medium | Breast Cancer | 259 | 1.19 | 44 |
| Prism/TiO2/Au/graphene/sensing medium | cancerous cells in the Adrenal Gland (PC12) | 282.86 | - | 45 |
| Prism/TiO2/Au/graphene/sensing medium | Breast MDA-MB-231 Cancer Detection | 292.85 | 0.263 | 41 |
| Prism/Ag/Bi2Te3/MXene/sensing medium | cancerous (skin, cervical and blood) cells | 319.46 | 0.30 | 74 |
| Prism/Ag/ZnSe/sensing medium |
MDA-MB-231 cells Cancerous HeLa cells |
359 247 |
0.22 0.26 |
42 |
| CaF2/Ag/Al2O3/Ag/graphene/analyte | Blood cancer | 427.43 | - | 46 |
| Prism/Ag/BaTiO3/Ag1/(MoSe2/WS2) |
breast (MM-231) cancer cells Skin cancer, Basal cells |
309.28 253.5 |
0.829 | 75 |
| Prism/Au/Gr/Ag/black P | Jurkat cancer cell | 329.1 | 76 | |
| Prism/Ag/InP/MXene/WS2/sensing medium | MCF-7 (Breast-II cancer cell) | 263.57 | 37 | |
| Prism/ZnO/Ag/Si3N4/WS2/sensing medium |
Blood cancer (Jurkat cell) Cervical cancer (HeLa cell) |
342.14 315.83 |
0.364 0.411 |
Present Work |
Distribution of electrical field in the proposed SPR biosensor
The SPR phenomenon occurs at the interface between the TMDCs and the sensing medium or analyte layer due to the matching of the momentum of the incident light and the surface plasmon waves. This equality, in the metal layer, will lead to the collective oscillation of the free electrons. The electric field of the waves of surface plasmon and the electric field of incident light are coupled so intensity of the electric field at the metal-dielectric interface will increase. As a result, the reflectance or transmission spectrum of the structure changes which is due to the interaction between the surface plasmon waves and the analyte layer. Using COMSOL multiphysics software the distribution of the electric field is simulated. As shown in Fig. 9, the interpretation of the performance parameters of the designed SPR biosensor with better sensitivity can be prospected by the distribution of electric field intensity from the prism boundary. To compare the proposed structure in SPR and non-SPR conditions, the electric field intensity propagation at the resonance and non-resonance angle is presented in Fig. 9a,b. In the non-resonance condition, no electric field intensity on the plasmonic layer is observed, whiles in the resonance condition, significant increase in the electric field intensity is observed at the sensing surface due to the maximum excitation of surface plasmons and the strong localization in the plasmonic layer.
Fig.9.
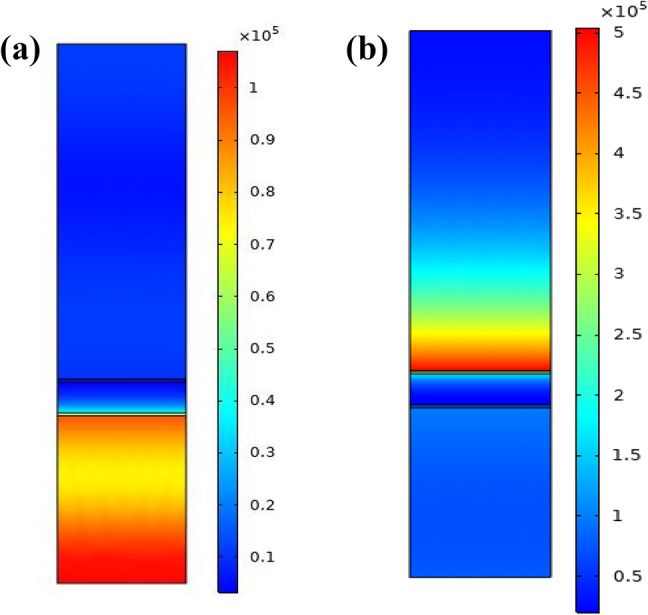
Electric field distribution (a) at a non-resonance angle (b) at a resonance angle for BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III) SPR biosensor.
Considering Fig. 9 the intensity of the electric field, at the interface between the TMDCs and the sensing medium is high due to the strongest excitation of surface plasmons which happen at the interface. In other words, since the interface between the TMDCs material and the sensing medium is conductive for detection of the refractive index variation of the sensing medium, so it can be seen that the electromagnetic wave energy is concentrated on this interface.
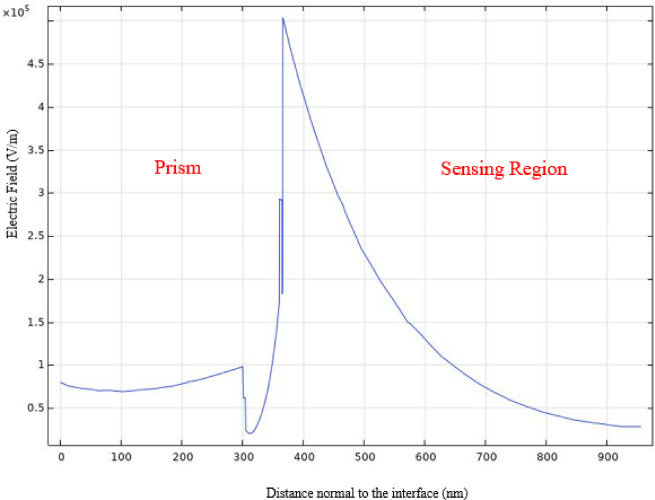
To further confirm the strong SPR excitation of the proposed BK7/ZnO/Ag/Si3N4/WS2/sensing medium (structure III), we employed the electric field distribution of the structure at the resonance angle condition. Thus, in Fig. 10, the electric field propagation versus distance normal to interface at the resonance angle is shown. As it is comprehended, an electric field enhancement is produced on the plasmonic Ag layer, and the intensity of the electric field exponentially falls to the sensing medium, which contains the biomolecules, so presents highly sensitivity to biomolecules interactions. It indicates the intensified field strength under these conditions, as it is common in resonance phenomena. The trend of EF is also consistent with the trend of the reflectance variation and sensitivity of the structure III.
Fig.10.
Cross-section plot of the electric field distribution along the direction perpendicular to the prism base at the resonance angle presenting an obvious evanescent field at the sensing interface.
Conclusion
In this study, we presented and theoretically analyzed the performance of a heterostructure composed of Ag-ZnO-Si3N4-TMDCs as an SPR sensor layers for detection of three different types of cancer cells, Jurkat, Basal and HeLa. Each material, ZnO, Si3N4 and TMDCs, was selected based on their specific and unique properties that contribute to enhancing the performance of the biosensor. Zinc Oxide (ZnO), a semiconductor, offers several key features beneficial for SPR biosensing including biocompatibility, a high refractive index, capability of light gathering plasmonic effect enhancement, surface functionalization capability and cost-effectiveness, making it a suitable choice for integration into SPR biosensor structure47,48. Likewise, dielectric Si3N4 was incorporated due to its specific advantages such as large RI which can improve light interaction in SPR sensor, chemical and thermal stability and some other properties which are essential for improvement of performance of the optical biosensor54–56. Moreover, Transition Metal Dichalcogenides (TMDCs) were chosen as the sensing layer owing to their favorable properties such as their adsorption ability, a large real part of dielectric constant and also their ability to support enhanced surface plasmon excitations, all of which significantly improve sensor performance. The average sensitivity for the structure BK7/ZnO/Ag/Si3N4/WS2/sensing medium of 342.14 deg/RIU for Jurkat cell detection which causes blood cancer, the value of 236 deg/RIU and 315.83 deg/RIU for Basal and HeLa cancerous cells, respectively, can be achieved from our design. These values demonstrate the high effectiveness of the design in detecting various types of cancerous cells. In addition, other performance parameters Figure of Merit (FOM), Detection Accuracy (DA) and Signal-to-Noise Ratio (SNR) of proposed structure are found to be superior in comparison with other configurations. It is concluded that WS2, in comparison to MoS2, MoSe2 and WSe2 gives rise to a greater shift in the resonance angle, resulting in the sensitivity enhancement for cancer cells detection. Besides, using finite element method (FEM) simulations, the electric field distribution at various layer interfaces is analyzed for BK7/ZnO/Ag/Si3N4/WS2/sensing medium configuration of proposed SPR sensor. The findings of this study provide valuable insights for designing of photonic and plasmonic biosensors, particularly for applications in cancer diagnostics. Totally, optical and plasmonic sensors can provide a promising opportunity for early and accurate cancer detection, offering a powerful tool in the fight against the cancer. Meanwhile, further research is still needed to optimize and improve the performance of optical sensors for broader use in medical and clinical applications.
Author contributions
E.J and S. A. developed the theory and presented idea. E.J and B.M. and S.A. performed the computational framework, simulations, calculations and modeling. All authors discussed the results and analyzed the data. S.A wrote the manuscript in consultation with B.M and S.A.supervised the project.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soni, G. K., Manhas, P. & Sharma, R. K. Peptide-based optical biosensors: a promising approach for early-stage cancer detection. Biosens. Bioelectronics: X. 12, 100259 (2022). [Google Scholar]
- 2.Du, Y. et al. Breast cancer early detection by using Fourier-transform infrared spectroscopy combined with different classification algorithms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.283, 121715 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Xiao, G. et al. Light-addressable photoelectrochemical sensors for multichannel detections of GPC1, CEA and GSH and its applications in early diagnosis of pancreatic cancer. Sens. Actuators B. 372, 132663 (2022). [Google Scholar]
- 4.Schiffman, J. D., Fisher, P. G. & Gibbs, P. Early detection of cancer: past, present, and future. Am. Soc. Clin. Oncol. Educational Book.35 (1), 57–65 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Yasli, A. Cancer detection with surface plasmon resonance-based photonic crystal fiber biosensor. Plasmonics16, 1605–1612 (2021). [Google Scholar]
- 6.Swami, S., Kayenat, F. & Wajid, S. SPR biosensing: cancer diagnosis and biomarkers quantification. Microchem. J.197, 109792 (2024). [Google Scholar]
- 7.Sahoo, P. R., Swain, P., Nayak, S. M., Bag, S. & Mishra, S. R. Surface plasmon resonance based biosensor: A new platform for rapid diagnosis of livestock diseases. Veterinary World. 9 (12), 1338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janze, E. J. T., Meshginqalam, B. & Alaei, S. A highly sensitive surface plasmon resonance biosensor using heterostructure of franckeite and TMDCs for Pseudomonas bacteria detection. Opt. Lasers Eng.181, 108404 (2024). [Google Scholar]
- 9.Wang, Y. et al. Investigation of phase SPR biosensor for efficient targeted drug screening with high sensitivity and stability. Sens. Actuators B. 209, 313–322 (2015). [Google Scholar]
- 10.Tabasi, O. & Falamaki, C. Recent advancements in the methodologies applied for the sensitivity enhancement of surface plasmon resonance sensors. Anal. Methods. 10 (32), 3906–3925 (2018). [Google Scholar]
- 11.Akjouj, A. & Mir, A. Performance evaluation of multifunctional SPR bimetallic sensor using hybrid 2D-nanomaterials layers. Optik269, 169857 (2022). [Google Scholar]
- 12.Jiang, Z. et al. The simultaneous detection of free and total prostate antigen in serum samples with high sensitivity and specificity by using the dual-channel surface plasmon resonance. Biosens. Bioelectron.62, 268–273 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Haseley, S. R., Talaga, P., Kamerling, J. P. & Vliegenthart, J. F. Characterization of the carbohydrate binding specificity and kinetic parameters of lectins by using surface plasmon resonance. Anal. Biochem.274 (2), 203–210 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Breault-Turcot, J., Poirier-Richard, H. P., Couture, M., Pelechacz, D. & Masson, J. F. Single chip SPR and fluorescent ELISA assay of prostate specific antigen. Lab. Chip. 15 (23), 4433–4440 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Mahani, M. et al. LSPR biosensing for the early-stage prostate cancer detection using hydrogen bonds between PSA and antibody: molecular dynamic and experimental study. J. Mol. Liq.324, 114736 (2021). [Google Scholar]
- 16.Uniyal, A. et al. Silver, silicon, and selenium-based surface plasmon resonance sensor for pathogen bacteria detection in visible region. Opt. Quant. Electron.57 (3), 196 (2025). [Google Scholar]
- 17.Tamang, J. S., Dhar, R. S., Uniyal, A. & Bhoi, A. K. A numerical method for developing an N-plasmonic sensor for biological sensing applications. Journal Optics, 1–10. (2025).
- 18.Basak, C., Islam, M. S., Hosain, M. K. & Kouzani, A. Z. An ultra-sensitive surface plasmon resonance biosensor with PtSe2 and BlueP/WS2 heterostructure. Heliyon, 10(19). (2024). [DOI] [PMC free article] [PubMed]
- 19.Daher, M. G. et al. An optical surface plasmon resonance device (OSPRD) for the accurate detection of injurious heavy metals. Plasmonics, 1–11. (2025).
- 20.Daher, M. G., Taya, S. A., Faragallah, O. S., AlZain, M. A., Almawgani, A. H., Alzahrani,A., … Zyoud, S. H. Supersensitive Novel Detector using Surface Plasmon Resonance Nanostructure Based on Black Phosphorus/Graphene for the Discovery of Various Pathogens in Drinking Water. Plasmonics, 1–9. (2024)
- 21.Daher, M. G. et al. Supersensitive detection of vibrio cholera using novel graphene-based optical device based on a surface plasmon resonance structure. Plasmonics19 (5), 2753–2760 (2024). [Google Scholar]
- 22.Finch, A., Metcalfe, K., Lui, J., Springate, C., Demsky, R., Armel, S., … Narod,S. (2009). Breast and ovarian cancer risk perception after prophylactic salpingo-oophorectomy due to an inherited mutation in the BRCA1 or BRCA2 gene. Clinical genetics, 75(3), 220–224. [DOI] [PubMed]
- 23.Carrascosa, L. G., Calle, A. & Lechuga, L. M. Label-free detection of DNA mutations by SPR: application to the early detection of inherited breast cancer. Anal. Bioanal. Chem.393, 1173–1182 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Hossain, M. B., Akib, T. B. A., Abdulrazak, L. F. & Rana, M. M. Numerical modeling of graphene-coated fiber optic surface plasmon resonance biosensor for BRCA1 and BRCA2 genetic breast cancer detection. Opt. Eng.58 (3), 037104–037104 (2019). [Google Scholar]
- 25.Uniyal, A., Ansari, G., Kumba, K., Karki, B. & Pal, A. An optimized design of SPR sensor with TiSi2/MXene/CNT multilayer structures: a TMM reflectance study using angle interrogation for haemoglobin detection. In Metaheuristics-Based Materials Optimization (433–454). (Woodhead Publishing, 2025).
- 26.Ansari, G., Oweis, R., Baldaniya, L., Barakat, M., S, R. J., Kaur, I., … Uniyal,A. (2025). Early detection of chikungunya virus using silver and zinc selenide multilayer structure utilizing the surface plasmon resonance: A numerical approach. Plasmonics, 1–12.
- 27.Basak, C., Hosain, M. K., Islam, M. S. & Kouzani, A. Z. Design and modeling of an angular interrogation based surface plasmon resonance biosensor for dengue virus detection. Opt. Quant. Electron.55 (5), 438 (2023). [Google Scholar]
- 28.Chang, C. C. et al. High-sensitivity detection of carbohydrate antigen 15 – 3 using a gold/zinc oxide thin film surface plasmon resonance-based biosensor. Anal. Chem.82 (4), 1207–1212 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Chen, S. et al. Combined detection of breast cancer biomarkers based on plasmonic sensor of gold nanorods. Sens. Actuators B. 221, 1391–1397 (2015). [Google Scholar]
- 30.Loyez, M. et al. HER2 breast cancer biomarker detection using a sandwich optical fiber assay. Talanta221, 121452 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Špringer, T. & Homola, J. Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma. Anal. Bioanal. Chem.404, 2869–2875 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Daher, M. G. et al. Optical detection of urea level in blood using novel SPR sensor employing silicon and tungsten diselenide nanomaterial for the early diagnosis of heart and kidney diseases. Plasmonics, 1–10. (2024).
- 33.Ayyanar, N., Raja, G. T., Sharma, M. & Kumar, D. S. Photonic crystal fiber-based refractive index sensor for early detection of cancer. IEEE Sens. J.18 (17), 7093–7099 (2018). [Google Scholar]
- 34.Yang, Y., Xiang, Y. & Qi, X. Design of photonic crystal biosensors for cancer cell detection. Micromachines14 (7), 1478 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veluchamy, D., Rajan, M. S. M. & Prajapati, Y. K. Enhancing Breast Cancer Biomarker Detection with a Portable Biosensor Utilizing Flower Core Photonic Crystal Fiber Architecture. Plasmonics, 1–13. (2024).
- 36.Ramanujam, N. R. et al. Enhanced sensitivity of cancer cell using one dimensional nano composite material coated photonic crystal. Microsyst. Technol.25, 189–196 (2019). [Google Scholar]
- 37.Uniyal, A., Pal, A., Ansari, G. & Chauhan, B. Numerical simulation of inp and MXene-Based SPR sensor for different cancerous cells detection. Cell Biochem. Biophysics, 1–14. (2025). [DOI] [PubMed]
- 38.Sharma, P., Sharan, P. & Deshmukh, P. A photonic crystal sensor for analysis and detection of cancer cells. In 2015 International conference on pervasive computing (ICPC) (pp. 1–5). IEEE. (2015), January.
- 39.Jabin, M. A. et al. Surface plasmon resonance based titanium coated biosensor for cancer cell detection. IEEE Photonics J.11 (4), 1–10 (2019). [Google Scholar]
- 40.Parvin, T., Ahmed, K., Alatwi, A. M. & Rashed, A. N. Z. Differential optical absorption spectroscopy-based refractive index sensor for cancer cell detection. Opt. Rev.28, 134–143 (2021). [Google Scholar]
- 41.Mostufa, S., Akib, T. B. A., Rana, M. M. & Islam, M. R. Highly sensitive TiO2/Au/graphene layer-based surface plasmon resonance biosensor for cancer detection. Biosensors12 (8), 603 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-assar, M., Taha, T. E., El-Samie, F. E. A., Fayed, H. A. & Aly, M. H. ZnSe-based highly-sensitive SPR biosensor for detection of different cancer cells and urine glucose levels. Opt. Quant. Electron.55 (1), 76 (2023). [Google Scholar]
- 43.Karki, B., Uniyal, A., Pal, A. & Srivastava, V. Advances in surface plasmon Resonance-Based biosensor technologies for cancer cell detection. Int. J. Opt.2022 (1), 1476254 (2022). [Google Scholar]
- 44.Kumar, A., Kumar, A., Dubey, S. K., Yadav, P. K. & Srivastava, S. K. Highly sensitive detection of carcinogenic biomarkers MCF-7 using graphene oxide-based SPR biosensor. Diam. Relat. Mater.139, 110321 (2023). [Google Scholar]
- 45.Kumar, V., Raghuwanshi, S. K. & Kumar, S. Detection of early-stage cancer in adrenal gland (PC12) cells using a prism-based SPR biosensor. In Plasmonics in Biology and Medicine XXI (Vol. 12860, 16–22). SPIE. (2024), March.
- 46.Kumar, R., Kushwaha, A. S., Srivastava, M., Mishra, H. & Srivastava, S. K. Enhancement in sensitivity of graphene-based zinc oxide assisted bimetallic surface plasmon resonance (SPR) biosensor. Appl. Phys. A. 124, 1–10 (2018). [Google Scholar]
- 47.Sexton, B. A., Feltis, B. N. & Davis, T. J. Characterisation of gold surface plasmon resonance sensor substrates. Sens. Actuators A: Phys.141 (2), 471–475 (2008). [Google Scholar]
- 48.Singh, Y. et al. High-performance plasmonic biosensor for blood cancer detection: achieving ultrahigh figure-of-merit. Plasmonics, 1–9. (2024).
- 49.Li, Y., Liao, Q., Hou, W. & Qin, L. Silver-based surface plasmon sensors: fabrication and applications. Int. J. Mol. Sci.24 (4), 4142 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maier, S. A. Plasmonics: Fundamentals and Applications (Vol1p. 245 (springer, 2007).
- 51.Stutius, W. & Streifer, W. Silicon nitride films on silicon for optical waveguides. Appl. Opt.16 (12), 3218–3222 (1977). [DOI] [PubMed] [Google Scholar]
- 52.Tene, T., Coello-Fiallos, D., Robalino, M. D. L. P., Londo, F. & Gomez, C. V. The effect of MoS2 and Si3N4 in surface plasmon resonance biosensors for HIV DNA hybridization detection: A numerical study. Micromachines16 (3), 295 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra, A. C., Sharma, A. K., Lohia, P. & Dwivedi, D. K. Silicon nitride (Si3N4) leads to enhanced performance of silica-silver based plasmonic sensor for colorectal cancer detection under optimum radiation damping. Solid State Commun.387, 115533 (2024). [Google Scholar]
- 54.Ku, S. L. & Lee, C. C. Optical and structural properties of silicon nitride thin films prepared by ion-assisted deposition. Opt. Mater.32 (9), 956–960 (2010). [Google Scholar]
- 55.Blázquez, O., López-Vidrier, J., Hernández, S., Montserrat, J. & Garrido, B. Electro-optical properties of non-stoichiometric silicon nitride films for photovoltaic applications. Energy Procedia. 44, 145–150 (2014). [Google Scholar]
- 56.Mudgal, N., Saharia, A., Choure, K. K., Agarwal, A. & Singh, G. Sensitivity enhancement with anti-reflection coating of silicon nitride (Si 3 N 4) layer in silver-based surface plasmon resonance (SPR) sensor for sensing of DNA hybridization. Appl. Phys. A. 126, 1–8 (2020). [Google Scholar]
- 57.Zeng, S., Hu, S., Xia, J., Anderson, T., Dinh, X. Q., Meng, X. M., … Yong, K. T.(2015). Graphene–MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors.Sensors and Actuators B: Chemical, 207, 801–810.
- 58.Wu, L. et al. Sensitivity enhancement by using few-layer black phosphorus-graphene/TMDCs heterostructure in surface plasmon resonance biochemical sensor. Sens. Actuators B. 249, 542–548 (2017). [Google Scholar]
- 59.Li, Y., Chernikov, A., Zhang, X., Rigosi, A., Hill, H. M., Van Der Zande, A. M.,… Heinz, T. F. (2014). Measurement of the optical dielectric function of monolayer transition-metal dichalcogenides: MoS 2, Mo S e 2, WS 2, and WS e 2. Physical Review B, 90(20), 205422.
- 60.Wu, L. et al. Few-layer Ti3C2Tx mxene: A promising surface plasmon resonance biosensing material to enhance the sensitivity. Sens. Actuators B. 277, 210–215 (2018). [Google Scholar]
- 61.Wu, L. et al. Sensitivity improved SPR biosensor based on the MoS_2/Graphene–Aluminum hybrid structure. J. Lightwave Technol.35 (1), 82–87 (2017). [Google Scholar]
- 62.Hossain, B. et al. A highly sensitive surface plasmon resonance biosensor using SnSe allotrope and heterostructure of BlueP/MoS2 for cancerous cell detection. Optik252, 168506 (2022). [Google Scholar]
- 63.Karki, B., Pal, A., Singh, Y. & Sharma, S. Sensitivity enhancement of surface plasmon resonance sensor using 2D material barium titanate and black phosphorus over the bimetallic layer of au, ag, and Cu. Opt. Commun.508, 127616 (2022). [Google Scholar]
- 64.Shalabney, A. & Abdulhalim, I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photonics Rev.5 (4), 571–606 (2011). [Google Scholar]
- 65.Mekuye, B. & Abera, B. Nanomaterials: an overview of synthesis, classification, characterization, and applications. Nano Select. 4 (8), 486–501 (2023). [Google Scholar]
- 66.Tynell, T. & Karppinen, M. Atomic layer deposition of zno: a review. Semicond. Sci. Technol.29 (4), 043001 (2014). [Google Scholar]
- 67.Sani, M. H. & Khosroabadi, S. A novel design and analysis of high-sensitivity biosensor based on nano-cavity for detection of blood component, diabetes, cancer and glucose concentration. IEEE Sens. J.20 (13), 7161–7168 (2020). [Google Scholar]
- 68.Wang, H., Zhang, H., Dong, J., Hu, S., Zhu, W., Qiu, W., … Luo, Y. (2018). Sensitivity-enhanced surface plasmon resonance sensor utilizing a tungsten disulfide (WS2) nanosheets overlayer. Photonics Research, 6(6), 485–491.
- 69.Lopez-Sanchez, O., Lembke, D., Kayci, M., Radenovic, A. & Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol.8 (7), 497–501 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Tajik, S. et al. Transition metal dichalcogenides: synthesis and use in the development of electrochemical sensors and biosensors. Biosens. Bioelectron.216, 114674 (2022). [DOI] [PubMed] [Google Scholar]
- 71.Wu, M. et al. Synthesis of two-dimensional transition metal dichalcogenides for electronics and optoelectronics. InfoMat3 (4), 362–396 (2021). [Google Scholar]
- 72.Elías, A. L. et al. Controlled synthesis and transfer of large-area WS2 sheets:from single layer to few layers. ACS Nano. 7 (6), 5235–5242 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Arunkumar, R., Suaganya, T. & Robinson, S. Design and analysis of 2D photonic crystal based biosensor to detect different blood components. Photonic Sens.9, 69–77 (2019). [Google Scholar]
- 74.Singh, S. et al. Simulation study of reconfigurable surface plasmon resonance refractive index sensor employing bismuth telluride and MXene nanomaterial for cancer cell detection. Phys. Scr.98 (2), 025813 (2023). [Google Scholar]
- 75.Kumar, V., Raghuwanshi, S. K. & Kumar, S. Nanomaterial-based surface plasmon resonance sensing chip for detection of skin and breast cancer. Plasmonics19 (2), 643–654 (2024). [Google Scholar]
- 76.Gollapalli, R., Phillips, J. & Paul, P. Ultrasensitive surface plasmon resonance sensor with a feature of dynamically tunable sensitivity and high figure of merit for cancer detection. Sensors23 (12), 5590 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.