Abstract
Background
The lack of simple but effective and affordable diagnostic tools presents a challenge for the management of perinatal asphyxia, especially in low- to middle-income countries. Current diagnostic tools, such as arterial blood gas estimation, are expensive and not readily available at primary and secondary levels of care, where most cases of perinatal asphyxia are identified. This causes a delay in diagnosis. Perinatal asphyxia may have improved outcomes if there are cheaper, reliable, and more convenient diagnostic biomarkers that can aid early diagnosis leading to early initiation of treatment. This study assessed the utility of the urinary uric acid-to-urinary creatinine (UUA/UC) ratio in the diagnosis of perinatal asphyxia.
Methods
This study was conducted among 90 term newborns aged less than 24-hours delivered at delivered at the Enugu State University Teaching Hospital (ESUTH). In the study population, there were an equal number of asphyxiated and apparently healthy babies. Relevant maternal and neonatal histories were obtained, and physical examination was carried out for all enrolled newborns. Umbilical arterial blood was collected for blood gas analysis, and spot urine samples were collected and sent for uric acid and creatinine estimation. Relevant statistical tests were applied in the data analysis.
Results
The mean UUA/UC ratio was significantly greater in the asphyxiated group (2.41 ± 0.73) than among the control group (0.87 ± 0.29) (t = 13.129, p < 0.001). The accuracy of the UUA/UC ratio in diagnosing perinatal asphyxia had an area under the curve (AUC) of 0.978, implying that the test is accurate. The cut-off point that gives the best diagnosis of perinatal asphyxia was 1.54, with a sensitivity of 95.6%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 96%. Additionally, the UUA/UC ratio has a very strong positive correlation with the severity of hypoxic–ischaemic encephalopathy (HIE) (r = 0.843, p < 0.001).
Conclusion
The UUA/UC ratio is a good diagnostic marker of asphyxia and varies with the severity of encephalopathy. Accordingly, the urinary uric acid-to-creatinine ratio is recommended as a surrogate biomarker for the diagnosis of perinatal asphyxia.
Keywords: Perinatal asphyxia, Diagnosis, Urinary uric acid, Creatinine
Background
Perinatal asphyxia is a critical medical condition in newborns that significantly contributes to neonatal morbidity and mortality [1]. Despite important advances in perinatal care, perinatal asphyxia is a major public health concern with severe complications and high case fatality rates, particularly in developing countries due to poor health facilities [1, 2].
The events that initiate the cascade that eventually lead to the tissue injury seen in perinatal asphyxia are not usually involved in the propagation of injury. The resulting sequalae are irreversible and may cause long term morbidity and mortality in affected babies. Interventions targeted at aetiology are the mainstay of reducing the burden of perinatal asphyxia. Once the cascade has been initiated, interventions aimed at slowing down and limiting the progression of events leading to tissue injury are key to reducing the organ-system effects of perinatal asphyxia. However, there exists only a narrow therapeutic window for such interventions. Utilization of this therapeutic window requires prompt diagnosis.
The outcome of babies with perinatal asphyxia may improve if there are cheaper, reliable and more convenient diagnostic biomarkers that can aid early diagnosis allowing for prompt initiation of treatment. Early diagnosis is critical, as timely intervention can mitigate many of the long-term complications associated with asphyxia [3–5]. Unfortunately, this opportunity is often limited in low to middle income countries due to the lack of the necessary diagnostic facilities such as blood gas analysis, and non-affordability of these investigations in the few facilities where they are available. Consequently, respiratory pathologies such as asphyxia are only usually detected when they present with clinical features such as pale/bluish limbs, difficulty in breathing and apnoea at which point severe damage may have already occurred [6].
The hypoxic-ischaemic injury in perinatal asphyxia results in a cascade that leads to increased uric acid production and excretion. The increased production of uric acid to a relatively constant creatinine in perinatal asphyxia results in increased urinary uric acid-to-urinary creatinine (UUA/UC) ratio. Several studies have observed increased UUA/UC ratio in babies with perinatal asphyxia [7–10]. The UUA/UC has been found to peak in urine samples of asphyxiated babies within the first 24-hours of birth [7, 10, 11].
One of the earliest studies on Urinary Uric Acid/urinary creatinine ratio in perinatal asphyxia was carried out in Israel by Bader et al. [12] in 1989. In the study, the UUA/UC ratio was greater in the asphyxiated group (2.06 ± 1.12) than in the control group (0.64 ± 0.48) (p < 0.001). The positive predictive value of UUA/UC > 1.2 was 78%, and the negative predictive value was 72%. The sensitivity was 74%, and the specificity was 76%. [12] Yashwanth et al. [11]in Hyderabad, India, prospectively studied 100 asphyxiated neonates and 100 non-asphyxiated neonates and reported a mean UUA/UC of 2.599 ± 0.820 vs. 0.913 ± 0.249 in each group, respectively. The cut-off UUA/UC value of > 1.4 for perinatal asphyxia had 94% sensitivity with a specificity of 96%, a positive predictive value of 95.52%, a negative predictive value of 94.12%, and an accuracy of 95% [11].
Although the UUA/UC ratio may have a lower sensitivity and specificity than other proposed biomarkers of perinatal asphyxia, such as calcium-binding protein B (S100β), neuron-specific enolase (NSE), and glial fibrillary acidic protein (GFAP), these alternative markers are limited to diagnosing brain injury specifically. More importantly, their use presents significant practical challenges, particularly in resource-limited settings. Assays for S100β, NSE, GFAP, and blood gas typically require blood or cerebrospinal fluid (CSF) samples, which makes them invasive [13, 14]. Moreover, their accurate measurement demands highly skilled personnel, specialized training, and access to well-equipped, often tertiary-level laboratories [8]. These requirements make their routine use difficult and costly, which present substantial setbacks in primary and secondary health care facilities where most cases of perinatal asphyxia occur, especially in low-resource environments.
This study assessed the utility of the UUA/UC ratio in the diagnosis of perinatal asphyxia. These findings may aid early diagnosis to enable early initiation of treatment.
Methods
This was a hospital-based case–control study carried out at the Special Care Baby Unit (SCBU) and the Maternity ward of the Enugu State University Teaching Hospital (ESUTH), Parklane, Enugu. The study recruited 45 asphyxiated neonates admitted to the SCBU and 45 apparently healthy term neonates without perinatal asphyxia who were delivered in the labour ward and maternity theatre of ESUTH.
The diagnosis of perinatal asphyxia was based on the presence of at least two of the following findings: (i) fetal distress signs (heart rate of less than 100 beats/minute, late decelerations, or an absence of heart rate variability) in the last week of delivery; (ii) Non vigorous meconium-stained baby and respiratory depression, hypotonia, or bradycardia; (iii) Apgar score ≤ 4 at 1 min and ≤ 6 at five minutes; (iv) need for resuscitation at birth; or (v) pH of blood less than 7.20 or a base deficit of at least 12 mmoL/L during the first hour of life [15].
The inclusion criteria for the subjects were as follows: babies less than 24 h old with the following: (1) gestational age between 37 completed weeks and 42 weeks, (2) appropriate weight for gestational age, and (3) features of perinatal asphyxia [15].
The exclusion criteria for the subjects were as follows: Babies with features of perinatal asphyxia [15] but with the following characteristics: (1) congenital malformations such as ambiguous genitalia and neural tube defects, (2) mothers who had received magnesium sulfate or opioids within 4 h prior to delivery or who had undergone caesarean section under general anaesthesia, (3) mothers at risk of antepartum hypovolaemia, including placenta previa, placental abruption, kidney disease, and heart failure, or (4) birth outside ESUTH (outborn babies).
The inclusion criteria for the control group, which was composed of apparently healthy babies less than 24 h old without perinatal asphyxia [15] were as follows: (1) gestational age between 37 completed weeks and 42 weeks, and (2) appropriate weight for gestational age.
The exclusion criteria for the control group were as follows: Babies without any feature of perinatal asphyxia [15] but with the following characteristics: (1) congenital malformation, (2) mothers who received magnesium sulfate or opioids within 4 h prior to delivery or who undergone caesarean section under general anaesthesia, (3) mothers at risk of antepartum hypovolaemia, including placenta previa, placental abruption, kidney disease, and heart failure, and (4) birth outside ESUTH (outborn babies).
For each case recruited, a control was selected as the next non-asphyxiated newborn delivered at the same hospital, matched for gestational age and sex, and whose parents provided informed consent.
Method of data collection
A detailed material history, including age, place of domicile, socioeconomic class [16]birth events, Apgar score, sex and weight of the baby, were documented on the pretested proforma. The gestational age was calculated using the new Ballard scoring system.
Thorough physical examination with special emphasis on neurological examination was performed for all the neonates included in the study. The HIE of the asphyxiated babies was staged using the modified SARNAT and SARNAT staging.
Sample collection
The samples for the study included spot urine samples from both the subjects and controls and umbilical artery blood from the subjects. Blood samples for blood gas analysis were analysed using the Abbot i-STAT autoanalyzer. Urine samples were collected under strict aseptic conditions from the subjects and controls within the first 24 h of birth using a size-6 Foley urethral catheter. The urine sample was stored in a refrigerator between + 2 °C and + 8 °C until analysis, although for no more than six hours. Urinary uric acid was estimated using the Randox RX Daytona Automated Biochemistry Analyser, which applies the spectrophotometric uricase method. Urinary creatinine was estimated with the same instrument described above by using Jaffe’s alkaline picrate method. Both uric acid and creatinine were measured in mmol/L. The use of standardized autoanalyzers and the blinding of the laboratory personnel to the case/control status of the samples were ensured to eliminate potential bias.
Results
There were 45 asphyxiated and 45 non-asphyxiated babies who were correctly matched for gestational age and sex. The mean gestational age (weeks) and birth weight (kg) of the subjects and controls were 38.29 ± 0.87 vs. 38.33 ± 0.93 (p-0.815) and 3.12 ± 0.24 vs. 3.08 ± 0.21 (p-0.516), respectively.
Sociodemographic characteristics
Table 1 indicates that the majority of the subjects belonged to the lower socioeconomic class. A significantly greater number of subjects resided in rural areas (n = 21) than did the control group (n = 8). The median APGAR score at one minute (interquartile range [IQR]) was 4.00 (1.00) for the subjects and 8.00 (1.00) for the controls. At five minutes, the median APGAR scores were 7.00 (1.00) for the subjects and 10.00 (1.00) for the controls. These differences were statistically significant, with a p value of < 0.001.
Table 1.
Sociodemographic and clinical characteristics of the study population
| Subjects N = 45 n (%) |
Controls N = 45 n (%) |
Test statistic | p value | |
|---|---|---|---|---|
| Socioeconomic class |
 2 = 13.931 2 = 13.931 |
0.008 | ||
| I | 2 (4.4) | 7 (15.6) | ||
| II | 5 (11.1) | 13 (28.9) | ||
| III | 11 (24.4) | 14 (31.1) | ||
| IV | 16 (35.6) | 8 (17.8) | ||
| V | 11 (24.4) | 3 (6.7) | ||
| Domicile | n = 45(100.0) |
 2 = 8.598 2 = 8.598 |
0.003 | |
| Urban | 24 (53.3) | 37 (82.2) | ||
|
Rural Mean Gestational Age in weeks (SD) |
21 (46.7) 38.28 (0.86) |
8 (17.7) 38.33 (0.92) |
t = −0.23 | 0.81 |
| Mean birth weight in kg (SD) | 3.11 (0.24) | 3.08 (0.20) | t = 0.65 | 0.51 |
| Median birth APGAR score in one minute (IQR) | 4.00 (1.00) | 8.00 (1.00) | U = 1035.00 | < 0.001 |
| Median birth APGAR score in five minute (IQR) | 7.00 (1.00) | 10.00 (1.00) | U = 1041.00 | < 0.001 |
SD standard deviation, IQR interquartile range, U Mann‒Whitney U test
Comparison of the UUA/UC ratio between asphyxiated and non-asphyxiated babies
Table 2 shows that the mean (SD) urinary uric acid level of the subjects (4.79 ± 2.51) was significantly greater than that of the controls (1.53 ± 0.58) (t = 8.482, p < 0.001). Similarly, the mean UUA/UC ratio was significantly greater among the subjects (2.41 ± 0.73) than among the controls (0.87 ± 0.29) (t = 13.129, p < 0.001).
Table 2.
Comparison of UUA/UC ratios between subjects and controls
| Subject Mean ± SD |
Control Mean ± SD |
MD | 95% CI of MD | t value | p value | |
|---|---|---|---|---|---|---|
| Uric acid (mmol/L) | 4.79 ± 2.51 | 1.53 ± 0.58 | 3.26 | 2.49–4.02 | 8.482 | < 0.001 |
| Creatinine (mmol/L) | 1.97 ± 0.68 | 1.77 ± 0.36 | 0.20 | −0.02–0.43 | 1.789 | 0.077 |
| UUA/UC ratio | 2.41 ± 0.73 | 0.87 ± 0.29 | 1.54 | 1.31–1.77 | 13.129 | < 0.001 |
MD mean difference, CI confidence interval
Linear regression analysis of potential confounders of the UUA/UC ratio
Table 3 shows the linear regression analysis of the potential predictors of the UUA/UC ratio. The table shows that asphyxiation status was the most important predictor of the UUA/UC ratio, accounting for approximately 66.7% of the variance (β = 0.76, t = 11.18, p = < 0.001, R2 = 0.667) after controlling for other variables, such as socioeconomic class, sex, gestational age, birth weight, domicile of the participants and time of sample collection.
Table 3.
Summary of multivariate linear regression results of the predictors of UUA/UC ratio
| Dependent variable | Significant predictors | Standardized β coefficient | t-stat | p value | R2 |
|---|---|---|---|---|---|
| UUA/UC ratio | Asphyxiation status | 0.76 | 11.18 | <0.001 | 0.667 |
| Socioeconomic class | 0.05 | 0.77 | 0.43 | ||
| Domicile | 0.09 | 1.22 | 0.22 | ||
| Gestational Age | 0.01 | 0.20 | 0.84 | ||
| Birth weight | 0.02 | 0.38 | 0.70 | ||
| Time of sample collection | 0.07 | 1.15 | 0.25 | ||
| Sex | 0.09 | 1.52 | 0.12 | ||
| F-stat. = 30.71; df = 6, 83 | Prob(F-stat.) = < 0.001 | ||||
Detection of asphyxia using UUA/UC ratio
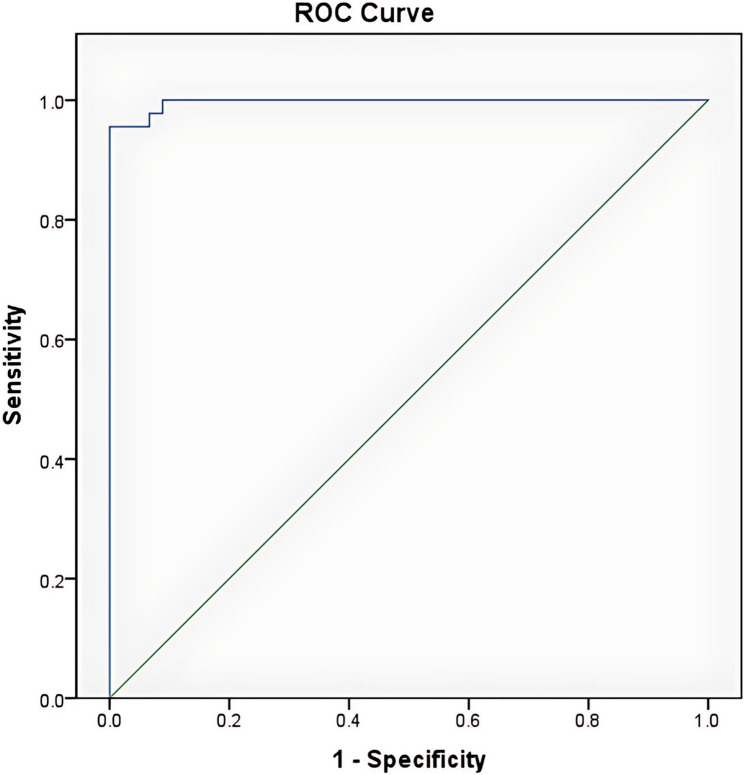
Figure 1 shows the receiver operating characteristic curve of the urinary uric acid and creatinine ratios vs. asphyxia status (asphyxiated/not asphyxiated). The area under the curve (AUC) (95%C.I.): 0.997 (0.990–1.000).
Fig. 1.
Receiver operating characteristic curve of the UUA/UC ratio among asphyxiated infants
As shown in Table 4, at a cut-off point of 1.54, the urinary uric acid-to-creatinine ratio was able to detect asphyxia among subjects with a sensitivity of 95.6%, a specificity of 100%, and both positive and negative predictive values of 100% and 96.0%, respectively. This cut-off point was established using the Younden’s index.
Table 4.
Sensitivity specificity positive predictive value and negative predictive value. Value of various cut-off points for the UUA/UC ratio for detecting asphyxia
| Asphyxia | ||||||||
|---|---|---|---|---|---|---|---|---|
| UUA/UC ratio |
Yes (n = 45) |
No (n = 45) |
Sensitivity | Specificity | Positive predictive value (PPV %) | Negative predictive value(NPV %) | ||
| True positive (TP) | False-negative (FN) | False-positive (FP) | True negative (TN) | |||||
| 1.3590 | 44 | 1 | 4 | 41 | 0.978 | 0.911 | 92 | 98 |
| 1.3814 | 44 | 1 | 3 | 42 | 0.978 | 0.933 | 94 | 98 |
| 1.4502 | 43 | 2 | 3 | 42 | 0.956 | 0.933 | 93 | 95 |
| 1.5003 | 43 | 2 | 2 | 43 | 0.956 | 0.956 | 96 | 96 |
| 1.5123 | 43 | 2 | 1 | 44 | 0.956 | 0.978 | 98 | 96 |
| 1.5368 | 43 | 2 | 0 | 45 | 0.956 | 1.000 | 100 | 96 |
| 1.5969 | 42 | 3 | 0 | 45 | 0.933 | 1.000 | 100 | 94 |
The majority of the subjects (29 (64.4%)) had mild HIE, 11 (24.4%) had moderate HIE, and 5 (11.1%) had severe HIE.
Comparison of the mean UUA/UC ratio with the severity of the hypoxic–ischaemic encephalopathy stages
The UUA/UC ratio increased significantly with increasing severity of hypoxic ischaemic encephalopathy (F = 94.18, p < 0.001). The mean ± SD urinary uric acid-to-creatinine ratios for HIE Stages I, II and III were 1.97 ± 0.29, 2.88 ± 0.21 and 3.91 ± 0.59, respectively. This finding revealed a significant difference between the stages of HIE and the UUA/UC ratio such that the more severe the HIE was, the higher the UUA/UC ratio was. The results of the Bonferroni post hoc correction revealed that this difference was between Stages I and II (p value < 0.001), Stages II and III (p value < 0.001) and Stages I and III (p value < 0.001). Additionally, there is a very strong positive correlation between the UUA/UC ratio and HIE stage (r = 0.843, p < 0.001).
Discussion
The mean UUA/UC ratio of asphyxiated neonates in this study was significantly greater than that of non-asphyxiated neonates (2.41 vs. 0.87). This was even true after controlling for potential confounding variables. The higher UUA/UC ratio among asphyxiated neonates than among non-asphyxiated neonates is reflected in the significantly higher urinary uric acid level. The higher UUA/UC ratio in subjects is attributable to the sequence of biochemical changes that take place in perinatal asphyxia, with excessive release of uric acid, which accumulates in the blood and is then excreted in the urine [17–19].
Uric acid, which is the end product of purine catabolism in primates, birds, and some other animals, increases during hypoxic–ischaemic injury following perinatal asphyxia [20]. This injury leads to cell death via necrosis and apoptosis [17]. During ischaemia, ATP is degraded, which leads to hypoxanthine accumulation [17]. Upon reperfusion, hypoxanthine is oxidized to xanthine and then to uric acid by xanthine oxidase, increasing uric acid levels in the blood due to tissue damage [18, 21, 22]. Approximately two-thirds of uric acid is eliminated by the kidneys, and one-third is eliminated by the gastrointestinal tract [9, 23]. Thus, increased uric acid production in perinatal asphyxia results in greater renal excretion. Creatinine is a natural by-product of muscle metabolism, formed through the spontaneous and irreversible conversion of creatine and creatine phosphate, which serve as immediate sources of high-energy phosphate bonds for ATP regeneration during muscle contraction [24]. Produced at a relatively constant rate depending on muscle mass, creatinine is distributed throughout total body water and is primarily excreted by the kidneys [24, 25]. It is freely filtered by the renal glomeruli, with minimal active secretion and negligible tubular reabsorption, and is unaffected by gastrointestinal bleeding or catabolic states [24, 25]. In perinatal asphyxia, increased ATP degradation leads to elevated uric acid production, whereas creatinine levels remain relatively stable. This results in an increased urinary uric acid-to-creatinine (UUA/UC) ratio, as observed in this study, and can serve as a useful marker of tissue hypoxia [8].
The finding of a significantly greater UUA/UC ratio in asphyxiated babies than in non-asphyxiated babies in this study is consistent with robust reports in the literature that support the UUA/UC ratio as a marker of perinatal asphyxia [7–9, 11, 26]. Given the significant difference observed in the UUA/UC ratio between asphyxiated and non-asphyxiated newborns, the index study upheld what has been known from previous studies that the UUA/UC ratio is a good marker of perinatal asphyxia.
The cut-off of ≥ 1.54 obtained in this study was highly sensitive and specific for the diagnosis of asphyxia. This performance is similar to that obtained by Yashwanth et al. [11] and Kumar et al. [10]. This finding, which is consistent with the increasing body of literature, suggests that the UUA/UC ratio is a promising diagnostic tool for perinatal asphyxia.
The index study revealed a significant relationship between the stages of encephalopathy and the UUA/UC ratio. There was a significant, strong positive linear relationship between the stages of hypoxic–ischaemic encephalopathy and the UUA/UC ratio. The stages of HIE reflect the severity of asphyxial insult to the brain and have been reported to correlate with the biochemical changes observed in perinatal asphyxia [7, 27, 28]. This worsening severity of HIE with progressive hypoxemia could account for the increasing UUA/UC ratio with advancing stages of encephalopathy. The increasing UUA/UC ratio with worsening severity of HIE obtained in this study is consistent with findings of numerous other studies, regardless of methodological differences [7, 8, 11, 27, 29, 30]. Siddharth et al. [27] collected urine samples up to 72 h of life, unlike other authors, who collected urine within the first 24 h of life [7, 8, 11, 29, 30]. In addition, while others have recruited term neonates, Ali et al. [30] recruited both term and preterm babies.
Furthermore, the significance of the variations in the UUA/UC ratio with the severity of HIE was observed to be between Stages I and II, Stages II and III, and Stages I and III. This finding agrees with previous studies except for the study by Erdag et al. [31]which revealed that there was no significant difference between HIE Stages I and II, probably because of the very small sample size used. [31]
Reviewing the UUA/UC ratio among the subjects and controls with respect to sex, the index study revealed no sex predilection for asphyxia. This may have occurred because the participants were correctly matched by sex, and the relationship between the UUA/UC ratio and sex may not be reliable. This was unlike an earlier study from the same centre by Ekwochi et al. [1]which reported a higher prevalence of perinatal asphyxia among males.
In addition, the index study revealed that the proportions of subjects in the upper socioeconomic class and domiciled in the urban area were significantly lower than those of the controls in the same socioeconomic class and place of domicile. This finding is similar to the findings of Ogunfowora and Ogunlesi [32] and Aliyu et al. [33]who reported that lower socioeconomic status and living in rural areas are risk factors for perinatal asphyxia. Although these variations are noted in the index study between the subjects and controls, they may not have a direct causal effect on the higher UUA/UC values among the subjects. Additionally, similar studies on the UUA/UC ratios in perinatal asphyxia have not reported that either socioeconomic class or place of domicile has any effect on the UUA/UC ratios.
Cost is a critical determinant of health outcomes in neonatal care, particularly in low- and middle-income countries (LMICs), where high out-of-pocket expenses are common. [34] A study by Ekwochi et al. [34] revealed that 100% of medical bills in the neonatal unit were out-of-pocket with 97.2% of these constituting catastrophic health expenditures, defined as expenses exceeding 10% of a household’s total monthly income [35]. Within this context, the choice of diagnostic equipment has major implications for affordability and sustainability. For example, the Abbott Point-of-Care (POC) blood gas analyser and its single-use CG8 + cartridge, which were used in this study, have capital costs of approximately US$18,999 (₦29, 410,452) and US$14.40 (₦22,291), respectively [36]making it a relatively expensive option for routine use in low-resource settings. (Dollars to Naira exchange rate: $1 = N1,548) [37]. In contrast, the Randox RX Daytona Automated Biochemistry Analyser used in this study presents a substantially more cost-effective alternative. With a capital cost of US$9,999 (₦15,478,452) [38]the Daytona system allows for combined uric acid and creatinine testing at just US$3.23 (₦5,000) per sample. These cost disparities underscores the urgent need for policymakers in LMICs to adopt cost-effective diagnostic tools that ensure broader access without exacerbating financial hardship among vulnerable families.
Conclusion
This study revealed that the UUA/UC ratio was significantly greater in asphyxiated babies than in non-asphyxiated babies. Additionally, at a cut-off of 1.54, the UUA/UC ratio had 100% specificity and 95.6% sensitivity in detecting perinatal asphyxia. Additionally, the UUA/UC ratio is positively correlated with the severity of encephalopathy and positively correlated with the UUA/UC ratio. Therefore, the UUA/UC ratio could serve as a promising diagnostic tool for perinatal asphyxia in resource-limited settings.
Limitations of the study
This study was conducted exclusively among term neonates; therefore, the findings may not be generalizable to preterm or postterm infants. Additionally, the single-centre design limits the external validity of the results, highlighting the need for multicentre studies to validate and extend these findings. The relatively small sample size may have constrained the ability to establish definitive UUA/UC cut-off values for identifying different stages of hypoxic–ischaemic encephalopathy (HIE). Furthermore, the study design did not include longitudinal follow-up, and as such, it was not possible to assess or predict long-term neurological outcomes among the subjects.
Acknowledgements
The authors sincerely appreciate Mr. Uche Ikenna for his contributions to the data analysis and Mr. Kamah of the Chemical Pathology Laboratory of ESUT Teaching Hospital for the meticulous sample analysis.
Abbreviations
- ATP
Adenosine triphosphate
- AUC
Area under the curve
- CI
Confidence interval
- CSF
Cerebrospinal fluid
- ESUTH
Enugu State University Teaching Hospital
- GFAP
Glial Fibrillary Acidic Protein
- HIE
Hypoxic–ischaemic encephalopathy
- IQR
Interquartile range
- LMICs
Low- and middle-income countries
- MD
Mean difference
- NPV
Negative predictive value
- NSE
Neuron-specific enolase
- POC
Point of care
- PPV
Positive predictive value
- ROC
Receiver operating characteristic
- S100B
Calcium-binding protein B
- SCBU
Special care baby unit
- SD
Standard deviation
- UC
Urinary creatinine
- UUA
Urinary uric acid
Authors’ contributions
This study was performed in collaboration with all the authors. ICC conceptualized the study. ICC and INA drafted the methodology. ICC and INA supervised the data collection and reviewed the manuscript draft. ANI and IKN performed the statistical analysis and wrote the results. ICC and IKN wrote the discussion. INA and ANI wrote the abstract and contributed to the discussion. All authors reviewed the final manuscript.
Funding
No funding.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and as additional supporting files.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained before the commencement of the study from the Ethics and Research Committee of the ESUT Teaching Hospital, Parklane, Enugu, with reference number ESUTHP/C-MAC/RA/034/Vol.1/291. Written consent was obtained from the parents or legal guardians of the neonates after the details of the research, including the specimen collection, had been explained. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ekwochi U, Asinobi NI, Osuorah CDI, Ndu IK, Ifediora C, Amadi OF et al. Incidence and Predictors of Mortality Among Newborns With Perinatal Asphyxia: A 4-Year Prospective Study of Newborns Delivered in Health Care Facilities in Enugu, South-East Nigeria. Clin Med Insights Pediatr [Internet]. 2017;11:117955651774664. Available from: http://journals.sagepub.com/10.1177/1179556517746646 [DOI] [PMC free article] [PubMed]
- 2.Antonucci R, Porcella A, Pilloni MD. Perinatal asphyxia in the term newborn. J Pediatr Neonatal Individ Med. 2014 [Cited 11 Oct 2018];3(2). Available from: www.jpnim.com. Accessed 2018 Aug 25.
- 3.Malta DC, Duarte EC, Escalante JJC, de Almeida MF, Sardinha LMV, Macário EM, Monteiro RA, Neto OLM. Avoidable causes of infant mortality in brazil, 1997–2006: contribution to performance evaluation of the united National health system. Cad Saude Publica. 2010;26(3):481–91. [DOI] [PubMed] [Google Scholar]
- 4.Mehta A, Chawla D, Kaur J, Mahajan V, Guglani V. Salivary lactate dehydrogenase levels can provide early diagnosis of hypoxic-ischaemic encephalopathy in neonates with birth asphyxia. Acta Paediatr. 2015;104(6):236–40. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XH, Zhang BL, Guo SM, Wang P, Yang JW. Clinical significance of dynamic measurements of seric TNF-α, HMGBl, and NSE levels and aEEG monitoring in neonatal asphyxia. Eur Rev Med Pharmacol Sci. 2017;21(19):4333–9. [PubMed]
- 6.Onu CC, Ndiomu E, Kengni U, Precup D, Sant’anna GM, Alikor E, Opara D. Ubenwa: cry-based diagnosis of birth asphyxia. 31st conference on neural processing systems (NIPS 2017), Long Beach, CA, USA. Available from: https://arxiv.org/pdf/1711.06405.pdf. Accessed 2018 Aug 25.
- 7.Krishnana E, Ponnusamy V, Sekar SP. Study of urinary uric acid and creatinine ratio as a marker of neonatal asphyxia for babies born in a tertiary care hospital. Int J Res Med Sci. 2017;5(12):5418–23. [Google Scholar]
- 8.Kumar M, Jain N, Thora S, Kiran T. Urinary uric acid/creatinine ratio as an additional early marker for perinatal asphyxia. JMSCR. 2017;05(07):25137–25137. [Google Scholar]
- 9.Patel KP, Makadia MG, Patel VI, Nilayangode HN, Nimbalkar SM. Urinary uric acid/creatinine ratio - A marker for perinatal asphyxia. J Clin Diagn Res. 2017;11(1):08–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar D, Chaudhari PK, Kumar A, Kamal S. Urinary uric acid and creatinine ratio as a marker of perinatal asphyxia. IOSR-JDMS. 2016;15(6):13–5. [Google Scholar]
- 11.Yashwanth K, Babu MS, Srinivas K, Mohan CC, Aswani N, Rekha NA. A study of urinary uric acid to creatinine ratio in assessing the severity of birth asphyxia. IOSR J Dent Med Sci. 2017;16(10):69–76. [Google Scholar]
- 12.Bader D, Gozal D, Weinger-Abend M, Berger A, Lanir A. Neonatal urinary uric acid/ceratinine ratio as an additional marker of perinatal asphyxia. Eur J Pediatr. 1995;154(9):747–9. [DOI] [PubMed] [Google Scholar]
- 13.Naithani M, Simalti AK. Biochemical markers in perinatal asphyxia. J Nepal Paediatr Soc. 2011;31(2):151–6. [Google Scholar]
- 14.Douglas-Escobar M, Weiss MD. Biomarkers of hypoxic-ischemic encephalopathy in newborns. Front Neurol. 2012;3(144):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boskabadi H, Omidian M, Tavallai S, Mohammadi S, Parizadeh M, Ghayour Mobarhan M, Ferns GAA. Serum Hsp70 antigen: early diagnosis marker in perinatal asphyxia. Iran J Pediatr 2015;25(2). e381. 10.5812/ijp.381. Available: https://pubmed.ncbi.nlm.nih.gov/26196004/ (Accessed 2019 Aug 25). [DOI] [PMC free article] [PubMed]
- 16.Ogunlesi A, Dedeke I, Kuponiyi O. Socio-economic classification of children attending specialist paediatric centres in Ogun state, Nigeria. Niger Med Pract. 2008;54(1):21–5. [Google Scholar]
- 17.Rainaldi MA, Perlman JM. Pathophysiology of birth asphyxia. Clin Perinatol. 2016;43(3):409–22. [DOI] [PubMed] [Google Scholar]
- 18.Porter KB, O’Brien WF, Benoit R. Comparison of cord purine metabolites to maternal and neonatal variables of hypoxia. Obstet Gynecol. 1992;79(3):394–7. [DOI] [PubMed] [Google Scholar]
- 19.Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32(4):260–7. [PubMed] [Google Scholar]
- 20.Boyle J. BookReview.In:NelsonD,andCoxM,editors.Lehningerprinciplesofbiochemistry,4thed.BiochemMolBiolEduc2005;33(1):74–5.
- 21.Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res. 1988;23(2):143–50. [DOI] [PubMed] [Google Scholar]
- 22.Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41(5):599–606. [DOI] [PubMed] [Google Scholar]
- 23.Manzke H, Kreudenstein PS, Dörner K, Kruse K. Quantitative measurements of the urinary excretion of creatinine, uric ucid, hypoxanthine and xanthine, uracil, cyclic AMP, and cyclic GMP in healthy newborn infants. Eur J Pediatr. 1980;133(2):157–61. [DOI] [PubMed] [Google Scholar]
- 24.Roger D. Creatininemetabolism:Reviewofliterature.Silo.Tips2016.Availablefrom:https://silo.tips/download/chapter-2-review-of-literature-2(Accessed:2019Sept20).
- 25.Hosten AO. BUN and Creatinine. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990. Chapter 193. PMID: 21250147 Available from: https://www.ncbi.nlm.nih.gov/books/NBK305/ (Accessed 2021 Nov 26).
- 26.Bhongir AV, Yakama AVV, Saha S, Radia SB, Pabbati J. The urinary uric acid/creatinine ratio is an adjuvant marker for perinatal asphyxia. Eur J Pharm Med Res. 2015;2(5):520–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Siddharth, Rai PL, Prasad PL. Urinary uric acid or urinary creatinine ratio as a non-invasive marker for perinatal asphyxia. Int J Contemp Pediatr. 2020;7(6):1378–82. [Google Scholar]
- 28.Michniewicz B, Szpecht D, Sowińska A, Sibiak R, Szymankiewicz M, Gadzinowski J. Biomarkers in newborns with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia. Child’s Nerv Syst. 2020;36(12):2981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary L, Palsania S, Berwal P, Sauparna C, Maheshwari A. Study of urinary uric acid and creatinine ratio as a marker of perinatal asphyxia and its correlation with different stages of hypoxic ischemic encephalopathy. J Pregnancy Child Health. 2017;04(03):1–4. [Google Scholar]
- 30.Ali E, Ali M, Elsayed LM, Abdo AAE, Mohamed NAE, Shehab MM. Urinary uric acid / creatinine ratio as a diagnostic marker for perinatal asphyxia. EJHM. 2021;85(1):3380–4. [Google Scholar]
- 31.Erdag GC, Vitrinel A. Can urinary uric acid/creatinine ratio be used as an additional marker for neonatal asphyxia? Int Pediatr. 2004;19(4):217–9. [Google Scholar]
- 32.Ogunfowora OB, Ogunlesi TA. Socio-clinical correlates of the perinatal outcome of severe perinatal asphyxia among referred newborn babies in Sagamu. Niger J Paediatr. 2020;47(2):110–8. [Google Scholar]
- 33.Aliyu I, Lawal T, Onankpa B. Prevalence and outcome of perinatal asphyxia: our experience in a semi-urban setting. Trop J Med Res. 2017;20(2):161–5. [Google Scholar]
- 34.Ekwochi U, Osuorah DC, Ndu IK, Ezenwosu OU, Amadi OF, Nwokoye IC, Odetunde OI. Out-of-pocket cost of managing sick newborns in Enugu, southeast Nigeria. Clinicoecon Outcomes Res. 2014;6:29–35. 10.2147/CEOR.S54674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. (2010). Health systems financing: The path to universal coverage. World Health Report. Available: https://iris.who.int/handle/10665/44371 (Accessed 2025 June 19). [DOI] [PMC free article] [PubMed]
- 36.MDMAXX. i-STAT handheld system critical blood analyser. Available: https://mdmaxx.com/products/abbott-04j6025-i-stat-handheld-system-critical-blood-analyzer?srsltid=AfmBOop0qdDZRjD71o_3-VAWl2wGadlhmjhtr4nMsolL8ZTDAg2mQ6LL (Accessed 2025 June 19).
- 37.Xe. Currency converter. Available: https://www.xe.com/en-gb/currencyconverter/convert/?Amount=1&From=USD&To=NGN (Accessed 2025 June 19).
- 38.Pinnacle Medical Equipment. Randox RX Daytona Chemistry Analyzer. Available: https://pinnaclemedequip.com/product/randox-rx-daytona-chemistry-analyzer/?srsltid=AfmBOoo1iVYMoU363I6IFv7tIlBD6NXEcKeE5hdIeRMfKiSEMAKkuzTA(Accessed 2025 June 19).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and as additional supporting files.