Abstract
Epilepsy affects over 50 million individuals worldwide. Despite the availability of anti-seizure medications, approximately 30% of patients remain drug-resistant, emphasizing the pressing need for alternative therapeutic strategies. In recent years, NMR-based metabolomics has emerged as a robust platform to investigate metabolic disturbances in neurological disorders, potentially improving diagnostic precision and explore individualized treatment options.In this study, we analysed serum metabolomics profiles from 32 patients with epilepsy, evaluated both at baseline and shortly after seizure episodes, and 28 healthy controls. Using an untargeted NMR-based metabolomics approach combined with multivariate and statistical analyses, we identified significant metabolic alterations and assessed their diagnostic potential. A total of 14 metabolites differed significantly between patients and controls. Among these, citrate, glutamate, proline, 3-methyl-2-oxovalerate, and glucose showed strong potential as biomarkers. Post-seizure samples revealed alterations in seven metabolites, including hippurate, pyroglutamate, isovalerate, creatinine, threonine, 3-methyl-2-oxovalerate, and 2-oxoisocaproate. Notably, we observed distinct metabolic signatures distinguishing focal from generalized seizures.To our knowledge, this is the first comprehensive serum metabolomics study using NMR to evaluate both basal and post-seizure states in epilepsy. Our findings highlight key alterations in energy metabolism, oxidative stress, and amino acid pathways, offering promising leads for improving clinical assessment and tailoring therapeutic strategies in epilepsy care.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-14718-1.
Keywords: NMR-metabolomics, Biomarkers, Epilepsy, Seizure-specific metabolic signatures
Subject terms: Biochemistry, Chemical biology, Neurology
Introduction
Epilepsy is a chronic neurological disorder characterized by recurrent spontaneous seizures, with an estimated worldwide prevalence of 50 million individuals1. The condition arises from a complex interplay of genetic, environmental, and physiological factors. In addition to its symptomatic seizures, epilepsy burdens individuals with a diverse array of psychological, social, and economic comorbidities2. Despite the prominence of anti-seizure medications (ASMs) in epilepsy management, a significant proportion of patients (around 30%) remain inadequately controlled, highlighting the urgent need for more effective treatments3–5. This emphasizes the critical necessity for deeper insights into the underlying mechanisms of this disorder, aiming to mitigate therapeutic gaps and optimize patient outcomes.
Addressing this challenge requires a deeper understanding of epilepsy’s metabolic underpinnings. Metabolomics, as a branch of systems biology, offers unique insights into these mechanisms6. By analysing small-molecule metabolites in biological systems, metabolomics can offer a window into the biochemical changes underlying epilepsy. Recent studies have pointed to disruptions in various metabolic pathways involved in seizure initiation, propagation, and resistance to ASMs7,8. Mapping these metabolic alterations not only contributes to elucidating disease mechanisms but also holds promise for improving diagnosis and guiding personalized interventions.
Among the array of analytical techniques utilized in metabolomics, nuclear magnetic resonance (NMR) spectroscopy emerges as a standout method due to its non-destructive nature, reproducibility, and its ability to generate quantitative profiles with minimal sample preparation9. When applied to epilepsy, NMR-based metabolomics can help uncover specific biochemical patterns associated with disease state and seizure activity, potentially facilitating better diagnostic and therapeutic strategies.
In this study, we conducted an untargeted NMR-based metabolomics analysis of serum samples collected from individuals with epilepsy, both at baseline state and immediately following seizure episodes, and compared them with samples from healthy controls. Our aim was to identify seizure-associated metabolic signatures and characterize biochemical pathways affected in epilepsy, with a focus on capturing dynamic changes related to seizure events.
Materials and methods
Study Design and Subject Selection
A total of 32 patients diagnosed with epilepsy (EP) and 28 healthy controls (HC) were prospectively enrolled between May 2021 and May 2023, from the Epilepsy Section at the Italian Hospital of Buenos Aires. Inclusion criteria for the EP group encompassed individuals aged over 18 years, admitted to the video-electroencephalography (Video-EEG) unit for 72 to 96 h, with epilepsy diagnosis confirmed through Video-EEG. Blood samples from basal-state epileptic patients (defined as having at least 24 h without seizures) and healthy controls were systematically collected after overnight fasting. Samples from the post-seizure group were obtained within 60 min after seizure onset, without regard to recent dietary intake, in order to capture early postictal metabolic alterations. None of the enrolled patients were undergoing ketogenic diet treatment at the time of the study.
Subjects in the HC group were individuals aged over 18 years, without an epilepsy diagnosis, free from known diseases, and not undergoing pharmacological treatment. This study was approved by the ethics committee of the Italian Hospital of Buenos Aires, facilitated through the PRIISA platform (Computerized Registry Platform for Health Research in Buenos Aires), with registration code 4553. All methods were performed in accordance with the relevant guidelines and regulations. Participants provided written informed consent prior to their inclusion in the study.
Demographic variables and details regarding epileptic seizures were collected for all participants. Epilepsy syndromes and seizures types were characterized in accordance with the classification and terminology of the International League Against Epilepsy. Additional parameters, including the presence or absence of refractory epilepsy, duration of disease progression, seizure frequency, temporal or extra-temporal localization for focal epilepsies, the presence or absence of abnormalities in brain magnetic resonance imaging, and details regarding the type of anti-seizure medications, were systematically determined. Seizure frequency was assessed using patient-reported seizure diaries over the past year and categorized into the following groups: (a) 1 seizure per day to 2 per week, (b) 1 seizure per week to 1 per month, (c) less than 1 seizure per month to 1 every 6 months, and (d) 1 seizure per year.
Sample collection
All blood samples were collected via venepuncture and processed immediately. Each sample was drawn into tubes containing 4 ml of activating gel and allowed to clot at room temperature. After visual confirmation of clot formation, samples were centrifuged at 3000 rpm for 5 min to separate the serum. The serum was aliquoted and promptly frozen at -80 °C. Samples exhibiting any signs of haemolysis were excluded from the study. In total, five samples were excluded for HC group.
For analysis, blood samples were categorized into three groups: (a) patients with serum samples collected under baseline conditions (n = 32), (b) patients with serum samples collected at post-seizure (n = 30), and (c) serum samples from healthy control subjects (n = 23).
Untargeted Nuclear Magnetic Resonance (NMR) Spectroscopy Metabolomics
Serum samples (300µL) were mixed with methanol in 1:2 ratios (v/v), vortexed, and incubated at − 20 °C for 20 min10. The mixtures were centrifuged at 13,400 rcf for 30 min to pellet proteins. Supernatants were decanted to fresh vials and dried using a centrifugal vacuum concentrator (SpeedVac). The dried samples were mixed with 500µL of phosphate buffer prepared in D2O (pH 7.40 ± 0.05), containing 3-trimethylsilyl-[2, 2, 3, 3, -2H4]-propionate (TSP) at a final concentration of 0.33 mM, and then transferred to 5 mm NMR tubes.
Sample sizes for NMR experiments were chosen using an analysis based approach, MetSizeR11. All NMR experiments were performed at 298 K on a Bruker Avance III spectrometer operating at a proton frequency of 600.1 MHz. Detailed experimental procedures are provided in Supporting Information.
Statistical analysis
Multivariate statistical analyses were performed to explore overall trends in the metabolic profiles and evaluate group discrimination. Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were conducted using the MetaboAnalyst 6.0 platform.
PCA was applied as an unsupervised method to reduce data dimensionality and visualize clustering patterns and potential outliers. PLS-DA, a supervised technique, was used to enhance group separation and identify the metabolites contributing most to the observed differences. Prior to analysis, the areas assigned to each metabolite in the NMR spectra (AUC) were normalized using probabilistic quotient normalization (PQN), and scaled using the Pareto method to minimize the influence of large-intensity variables while preserving data structure. Model performance was assessed through fivefold cross-validation. Variable importance in projection (VIP) scores were calculated to rank metabolites based on their contribution to group separation. Multivariate comparisons included: (a) epileptic patients in the basal state (n = 30) vs. healthy controls (n = 21), (b) basal (n = 30) vs. post-seizure states (n = 28) within the epilepsy group, and (c) focal (n = 18) vs. focal to bilateral tonic-clonic seizures (n = 8).
Statistical significance was evaluated by Wilcoxon rank-sum test, taking p < 0.05 as significant according to FDR (False Discovery Rate) and by one-way ANOVA and Tukey’s HSD post-hoc tests for multiple comparisons. Model performance was evaluated using cross-validated area under the receiver operating characteristic curve (AUROC).
Results
Demographic and Clinical Overview
Our study included 60 participants: 32 patients with epilepsy (EP) and 28 healthy controls (HC), with balanced gender distribution between both groups (50% female). No significant age differences were observed between the EP and HC groups (mean age: 33.7 years, SD = 12.0 vs. 33.6 years, SD = 9.9; p = 0.2), minimizing age as a confounding factor.
Of the 32 EP, 30 were diagnosed with focal epilepsy, with 67% presenting extra-temporal localization. The average disease duration was 19.0 years (SD = 12.9, range 1–40 years). Notably, 71.8% of the patients (n = 23) were classified as having refractory epilepsy, and more than half (53%) experienced a seizure frequency ranging from one per day to two per week. The median number of anti-seizure medications was 2.1 (range 1–4), with Levetiracetam being the most frequently prescribed (62.5%). Brain MRI findings were normal in 59% of patients (n = 19). During video-EEG monitoring, 28 patients experienced seizures: 75% (n = 21) had focal onset with impaired awareness (18 motor, 3 non-motor onset), while 25% (n = 7) had focal to bilateral tonic-clonic (FBTC) seizures.
In total, 88 serum samples were collected: 32 at baseline, 28 within 60 min post-seizure, and 28 from healthy controls.
Key Metabolic Biomarkers Distinguishing Epileptic Patients in Basal State from Healthy Controls
We first assessed the metabolic landscape of epileptic patients in their interictal (baseline) state relative to healthy individuals. Using an untargeted NMR-based approach, we identified and quantified 44 metabolites from a total of 52 detected (Figure S1, Table S1), excluding those with poor signal quality in the ¹H-NMR spectrum or technical artefacts such as residual methanol.
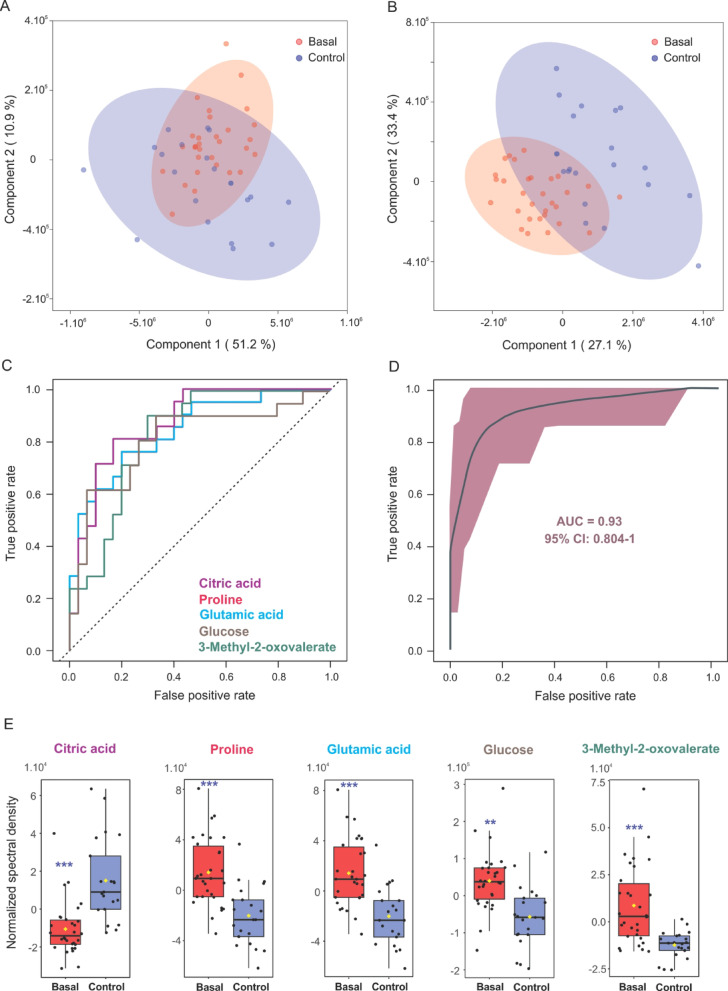
Principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) suggested a trend toward separation between EP and HC metabolic profiles (Fig. 1A and B, Table S2). Fourteen metabolites differed significantly (P < 0.05) between groups (Table 1). Notably, citric acid, 2-oxoisocaproic acid, acetylcarnitine, 3-hydroxybutyric acid, and glycerol were reduced in EP, while glutamic acid, proline, 3-methyl-2-oxovaleric acid, glucose, 2-hydroxybutyric acid, tyrosine, mannose, pyroglutamic acid, and carnitine were elevated.
Fig. 1.
Multivariate analysis and ROC performance of metabolites distinguishing epileptic patients in the basal state from healthy controls. (A) PCA score plot and (B) PLS-DA score plot for metabolic data from patients (red) and healthy controls (blue). The ellipses represent 95% confidence intervals for each group. (C) ROC curves for individual metabolites with area under the curve (AUC) values greater than 0.8. (D) Combined ROC curve for a multivariate model incorporating selected metabolites. (E) Box plots illustrating the distribution of metabolite levels between patients in the basal state (red) and healthy controls (violet), with each dot representing an individual measurement. ***: p < 0.0001; **: p < 0.001.
Table 1.
Serum differential metabolites in epileptic patients in the basal state compared to healthy controls. This table highlights metabolites with significant alterations in epileptic patients versus healthy controls. Each metabolite is presented with its false discovery rate (FDR) from Wilcoxon rank-sum test, area under the curve (AUC) values with 90% confidence intervals (CI), log2 fold-change values and PLS-DA VIP scores.
| Metabolite | Epileptic Patients vs. Controls | |||||
|---|---|---|---|---|---|---|
| FDR | AUC | 90% CI | Log 2 Fold-Change | VIP score | ||
| Citric acid | 0.0003 | 0.88 | 0.77–0.95 | -0.61 | 0.97 | |
| Glutamic acid | 0.0004 | 0.84 | 0.73–0.93 | 0.59 | 1.29 | |
| Proline | 0.0004 | 0.85 | 0.73–0.94 | 0.59 | 1.29 | |
| 3-Methyl-2-Oxovaleric acid | 0.0007 | 0.83 | 0.69–0.92 | 1.40 | 0.79 | |
| Glucose | 0.0011 | 0.81 | 0.69–0.93 | 0.24 | 3.64 | |
| 2-Hydroxybutyric acid | 0.0060 | 0.77 | 0.67–0.91 | 0.82 | 1.51 | |
| 2-Oxoisocaproic acid | 0.0079 | 0.77 | 0.67–0.91 | -0.23 | 0.86 | |
| Acetylcarnitine | 0.0209 | 0.74 | 0.66–0.90 | -0.40 | 0.75 | |
| Tyrosine | 0.0209 | 0.73 | 0.66–0.90 | 0.37 | 1.13 | |
| 3-Hydroxybutyric acid | 0.0359 | 0.72 | 0.65–0.89 | -0.92 | 0.86 | |
| Glycerol | 0.0453 | 0.71 | 0.64–0.88 | -0.30 | 0.63 | |
| Mannose | 0.0453 | 0.70 | 0.64–0.87 | 0.28 | 0.34 | |
| Pyroglutamic acid | 0.0453 | 0.70 | 0.64–0.87 | 0.60 | 0.40 | |
| Carnitine | 0.0453 | 0.70 | 0.64–0.87 | 0.18 | 0.91 | |
To assess the ability of these highlighted metabolites to discriminate between groups, we performed receiver operating characteristic (ROC) curve analysis (Fig. 1C). We identified a subset of metabolites that distinctly characterize epilepsy-associated metabolic changes, including citric acid, glutamic acid, proline, 3-methyl-2-oxovaleric acid and glucose (Fig. 1E and S2). Furthermore, these five metabolites jointly yield an AUC value of 0.93, with a 95% confidence interval (CI) of 0.80-1.00 and an average accuracy of 0.84 based on 100 cross validations (p < 0.002, Fig. 1D).
Additionally, pathway enrichment analysis further revealed that these alterations corresponded to disruptions in key metabolic routes, including the glucose-alanine cycle, glucose metabolism, beta-oxidation of very long-chain fatty acids, glutathione metabolism, transfer of acetyl groups into mitochondria, and the degradation pathways of branched-chain aminoacids (BCAA) (Figure S3).
Serum Metabolic Shifts Following Seizure Episodes
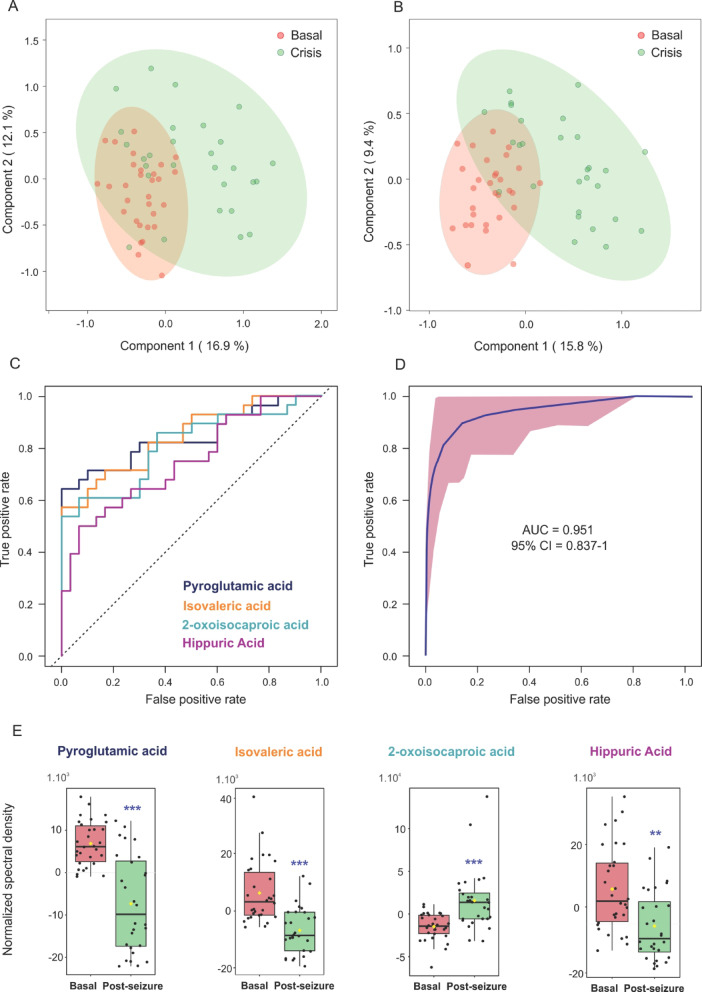
We subsequently explored the immediate metabolic consequences of seizure activity by comparing serum samples from EP at baseline and within 60 min post-seizure. Multivariate analysis using PCA and PLS-DA revealed a separation pattern with some degree of overlap between the two states (Fig. 2A and B, Table S2), with the first principal component capturing over 50% of the total variance. The PLS-DA model yielded robust statistical parameters (R2 = 0.82, Q2 = 0.60), supporting its discriminative power.
Fig. 2.
Multivariate analysis and ROC performance of metabolites distinguishing epileptic patients in the basal state from the post-seizure state. (A) PCA score plot and (B) PLS-DA score plot for metabolic data from patients in the basal state (red) and post-seizure (green). The ellipses represent 95% confidence intervals for each group. (C) ROC curves for individual metabolites with area under the curve (AUC) values greater than 0.8. (D) Combined ROC curve for a multivariate model incorporating selected metabolites. (E) Box plots illustrating the distribution of metabolite levels between patients in the basal (red) and post-seizure (green) states, with each dot representing an individual measurement. ***: p < 0.0001; **: p < 0.001.
Seven metabolites were found to differ significantly (p < 0.05) between baseline and post-seizure states (Table 2). Pyroglutamic acid, isovaleric acid, hippuric acid, 3-methyl-2-oxovaleric acid, creatinine, and threonine were significantly decreased post-seizure, while 2-oxoisocaproic acid was elevated.
Table 2.
Serum differential metabolites in epileptic patients in basal and post-seizure state. The table highlights metabolites with significant alterations in epileptic patients in basal and post-seizure state. Each metabolite is presented with its false discovery rate (FDR) from Wilcoxon rank-sum test, area under the curve (AUC) values with 90% confidence intervals (CI), log2 fold-change values and PLS-DA VIP scores.
| Metabolite | Basal vs. post-seizure state | ||||
|---|---|---|---|---|---|
| FDR | AUC | 90% CI | Log 2 Fold-Change | VIP score | |
| Isovaleric acid | 0.000005 | 0.846 | 0.733–0.926 | 2.12 | 1.91 |
| Pyroglutamic acid | 0.000005 | 0.842 | 0.741–0.930 | 1.08 | 3.08 |
| 2-Oxoisocaproic acid | 0.000038 | 0.811 | 0.683–0.918 | -0.45 | 1.32 |
| Hippuric acid | 0.008372 | 0.800 | 0.617–0.863 | 0.77 | 1.86 |
| 3-Methyl-2-Oxovaleric acid | 0.015174 | 0.730 | 0.582–0.846 | 0.91 | 1.45 |
| Creatinine | 0.026862 | 0.715 | 0.595–0.843 | 0.24 | 1.32 |
| Threonine | 0.049891 | 0.695 | 0.537–0.806 | 0.37 | 1.25 |
ROC analysis further identified pyroglutamic acid, isovaleric acid, 2-oxoisocaproic acid, and hippuric acid as high-performing markers of the post-seizure state (Fig. 2C and E and S4). Collectively, these four metabolites achieved an area under the curve (AUC) value of 0.95, with a 95% confidence interval (CI) of 0.837-1.000, and an average accuracy of 0.836 based on 100 cross-validations (p < 0.001) (Fig. 2D).
Pathway enrichment analysis of these differentially prevalent metabolites revealed significant perturbations in metabolic networks including branched-chain aminoacids degradation, glutathione metabolism, threonine and 2-oxobutanoate degradation, gluconeogenesis, pyruvate metabolism, and glycine and serine metabolism (Figure S5).
Distinct Metabolic Profiles by Seizure Type
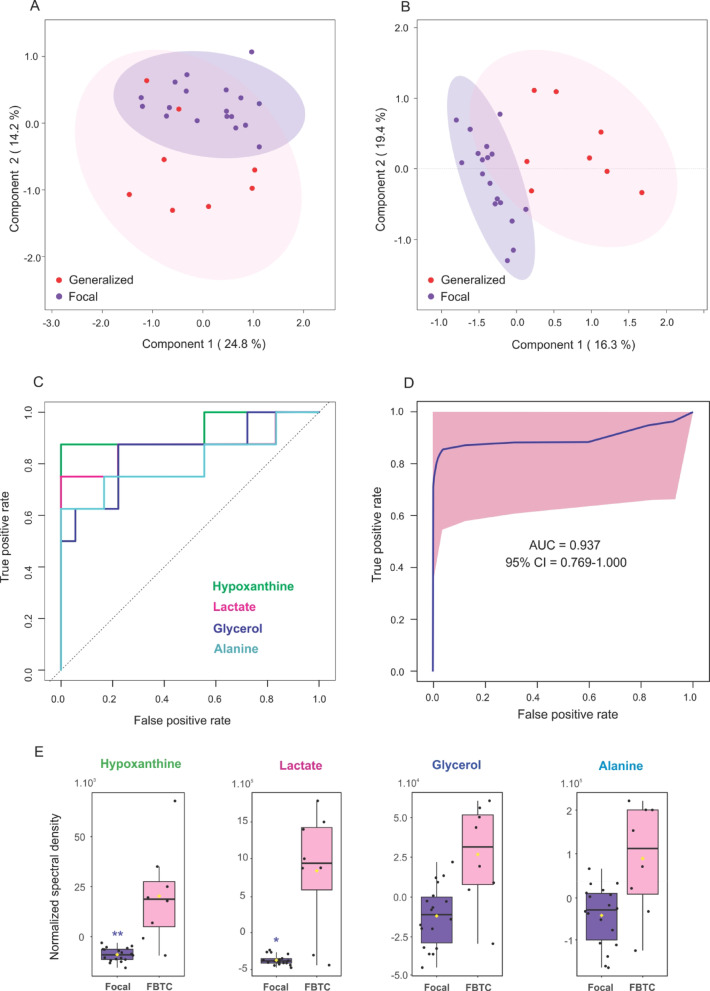
To investigate whether metabolic signatures varied by seizure phenotype, we stratified EP into focal and FBTC subgroups. Multivariate analyses using PCA and PLS-DA revealed a partial separation between the two subgroups (Fig. 3A and B, Table S2), with the first principal component accounting for nearly 25% of the variance. Two metabolites-lactic acid and hypoxanthine were found to be significant elevated in the FBTC group (p < 0.05, Fig. 3E; Table 3).
Fig. 3.
Multivariate analysis and ROC performance of metabolites distinguishing epileptic patients with focal versus focal to bilateral tonic-clonic seizures. (A) PCA score plot and (B) PLS-DA score plot for metabolic data from patients with focal (blue) and focal to bilateral tonic-clonic (FBTC) seizures (red). The ellipses represent 95% confidence intervals for each group. (C) ROC curves for individual metabolites with area under the curve (AUC) values greater than 0.8. (D) Combined ROC curve for a multivariate model incorporating selected metabolites. (E) Box plots illustrating the distribution of metabolite levels between patients with focal (purple) and FBTC (pink) seizures, with each dot representing an individual measurement.**: p < 0.001, *: p < 0.05.
Table 3.
Serum differential metabolites in epileptic patients with focal and focal to bilateral tonic-clonic seizures. This table highlights metabolites with significant alterations in epileptic patients with focal versus focal to bilateral tonic-clonic (FBTC) seizures. Each metabolite is presented with its false discovery rate (FDR) from Wilcoxon rank-sum test, area under the curve (AUC) values with 90% confidence intervals (CI), log2 fold-change values and PLS-DA VIP scores.
| Metabolite | Focal vs. FBTC seizures | ||||
|---|---|---|---|---|---|
| FDR | AUC | 90% CI | Log 2 Fold-Change | VIP score | |
| Hypoxanthine | 0.007661 | 0.93 | 0.79-1.00 | 2.85 | 2.11 |
| Lactate | 0.046865 | 0.87 | 0.65-1.00 | 2.31 | 3.22 |
Subsequent ROC analysis supported the predictive value of these metabolites (Fig. 3C, Figure S6). Notably, the combination of hypoxanthine and lactic acid achieved outstanding discriminant performance, with an AUC of 0.94 (95% CI: 0.769–1.000) and an average accuracy of 0.954 across 100 cross-validation (p < 0.001; Fig. 3D).
Discussion
Metabolic signatures in epileptic patients compared to healthy controls
Our comprehensive NMR-based metabolomics study reveals substantial metabolic alterations in the serum of EP compared to HC, unveiling significant disparities in 14 metabolites and providing new insights into the underlying metabolic dysregulation associated with epilepsy.
A key finding of our study is the significant reduction of citric acid levels in EP, likely reflecting impaired mitochondrial function and dysregulated energy metabolism. Given the central role of citrate in the tricarboxylic acid cycle, its depletion may signal compromised aerobic respiration, possibly driven by heightened oxidative stress or inflammation, two processes well documented in epileptogenesis7,12–15.
The consistent elevation of BCAA catabolites, particularly 2-oxoisocaproic acid (Ketoleucine) and 3-methyl-2-oxovaleric acid (KMV), suggests an increased reliance on amino acid metabolism to support energy production. This shift likely represents an adaptive response to chronic excitatory activity and the metabolic burden imposed by recurrent seizures, as previously proposed in models of refractory epilepsy16. In muscle tissue, transamination of BCAAs yields glutamic acid, which in turn contributes anaplerotic substrates (such α-ketoglutaric acid) to the Krebs cycle, while simultaneously generating NADH to fuel mitochondrial respiration16. Consistent with this metabolic reprogramming, our study revealed significantly elevated plasma glutamic acid levels in EP, which is in agreement with earlier reports of its accumulation in this population17.
Our data also revealed increased levels of proline in EP, which has been linked to several metabolic conditions, including type 2 diabetes, obesity, and cachexia18. High proline levels suggest a wider disruption of metabolic processes, often associated with insulin resistance and altered amino acid metabolism18. Although the precise role of proline in epilepsy remains unclear, its elevation may reflect a more general state of systemic stress.
Disruptions in lipid metabolism were also evident in EP. The observed decrease in acetylcarnitine and elevation in carnitine suggest impaired β-oxidation and possible compensatory upregulation of fatty acid transport mechanisms19–21. Additionally, elevated 3-hydroxybutyrate —an important ketone body—in EP, may indicate enhanced ketogenesis, likely serving as an alternative energy source in the context of increased energy demands or mitochondrial dysfunction22.
Altered glycerol levels in EP further support the presence of disrupted lipid mobilization and impaired energy homeostasis. The observed reduction in glycerol may reflect increased utilization for gluconeogenesis and ketogenesis, or a decrease in lipolytic activity, metabolic pathways that could all impact the ability to sustain energy production during or between seizures23.
Of particular interest is the modest yet significant elevation of glucose in EP. This finding likely reflect the convergence of multiple metabolic disturbances described above, most of which suggest a compensatory metabolic environment aimed at preserving energetic stability. In this context, the rise in glucose may indicate reduced cellular utilization, potentially secondary to insulin resistance or mitochondrial inefficiency. In addition, elevated mannose, a key monosaccharide involved in glycoprotein synthesis, could point to disrupted glycosylation processes or broader shifts in carbohydrate metabolism24.
The increase in EP of pyroglutamate, a by-product of glutathione metabolism, may indicate redox imbalance and a potential rise in oxidative stress burden25,26. Elevated 2-hydroxybutyrate levels in EP reinforce this notion, as this metabolite is primarily produced in the liver under oxidative stress to replenish glutathione. In line with this observation, 2-hydroxybutyrate is increasingly recognized as a biomarker of metabolic disturbances, particularly in insulin resistance and glucose intolerance27.
Tyrosine levels were significantly elevated in EP. As a precursor for catecholamines, tyrosine plays a critical role in neuronal signalling28. Additionally, since altered tyrosine metabolism has been linked to neuroinflammation29its elevation may reflect the dual involvement of neurotransmitter dysregulation and inflammatory pathways in the epileptic process.
Interestingly, a recent study on mesial temporal lobe epilepsy identified five altered metabolites when comparing patients to controls: glucose, saturated lipids, 3-hydroxybutyrate, isoleucine, and proline. These findings, which align with ours, suggest that these metabolic signatures may be broadly linked to epileptic seizures rather than being specific to a particular epilepsy syndrome30.
Serum Metabolic Changes Following Seizures
Analysing serum metabolic changes following seizures revealed key insights into the biochemical effects of epileptic episodes. Our study identified eight metabolites with significant alterations, indicating a complex metabolic response to acute neuronal activity.
The post-seizure decrease in pyroglutamic acid was particularly notable due to its role in glutathione metabolism, as mentioned earlier. This observation may reflect oxidative alterations that could potentially exacerbate neuronal injury or be involved in epilepsy pathophysiology31.
Isovaleric acid, a branched-chain fatty acid from leucine metabolism, was diminished post-seizure, suggesting increased BCAA catabolism during seizures, possibly for energy production or stress response. Similarly, the decrease in 3-methyl-2-oxovaleric acid, linked to BCAA metabolism, further supports this and indicates altered amino acid metabolism during epileptic activity32.
Hippuric acid, a glycine conjugate involved in liver detoxification, showed a decrease post-seizure, likely indicating a depletion of glycine, which also functions as a neurotransmitter in inhibitory signalling in the central nervous system33. This reduction may reflect an increased demand for glycine, not only for metabolic pathways but also for glutathione synthesis, as suggested by the elevated pyroglutamic acid levels.
The drop in creatinine levels post-seizure could indicate changes in muscle metabolism. As creatinine is a breakdown product of creatine phosphate, reduced levels suggest rapid depletion of muscle energy stores during seizures, consistent with increased overall energy demand34.
Threonine, an essential amino acid involved in protein synthesis and the production of glycine and serine, exhibited a decrease post-seizure. Glycine and serine are crucial neurotransmitters in the CNS and regulate excitatory-inhibitory balance. Consequently, the reduction in threonine might suggest an increased utilization of these neurotransmitters during the heightened excitatory state of seizures35.
During seizures, the brain’s surge in electrical activity increases energy demand, prompting the body to mobilize energy stores, including amino acids like leucine. The elevated levels of 2-oxoisocaproic acid indicate enhanced leucine degradation, suggesting a shift towards energy production pathways to support the heightened metabolic activity36.
The ROC analysis emphasized the predictive potential of metabolites such as pyroglutamic acid, isovaleric acid, 2-oxoisocaproic acid, and hippuric acid in distinguishing between basal and post-seizure states. These metabolites may have value as biomarkers for monitoring seizure activity and evaluating therapeutic efficacy. This could be particularly valuable in clinical settings requiring rapid assessment of seizure impact and recovery.
Distinct Metabolic Profiles in Focal and Focal to Bilateral Tonic-Clonic Seizures
Our stratified analysis by seizure type revealed that FBTC seizures are metabolically distinct from focal seizures. In FBTC seizures, elevated lactic acid points to a surge in anaerobic glycolysis driven by the intense energy demands and hypoxia characteristic of prolonged neuronal activity. Alongside this alteration in energy metabolism, FBTC seizures were characterized by a significant increase in hypoxanthine, a purine degradation product linked to nucleotide catabolism and oxidative stress37.
ROC analysis further identified hypoxanthine and lactate as robust biomarkers for distinguishing focal from FBTC seizures, demonstrating high predictive accuracy. These findings underscore the potential of metabolic profiling to improve the differential diagnosis of seizure types, paving the way to enhance personalized treatment strategies and better patient outcomes.
Conclusion
NMR-based metabolomics stands as a powerful asset in generating advancements in clinical research and identifying diagnostic and prognostic biomarkers. Optimized protocols for sample preparation, rapid analytical workflows, and outstanding reproducibility collectively reinforce the essential role of this method in metabolomics research. To the best of our knowledge, this study is the first to use untargeted NMR-based metabolomics to comprehensively analyse metabolic alterations in the serum of epileptic patients, both at baseline and immediately following a seizure, in comparison to a non-epileptic control group.
Significant differences in metabolites were identified between epileptic patients and healthy controls, highlighting disruptions in energy metabolism, amino acid metabolism, and neurotransmitter pathways. Post-seizure analysis revealed notable shifts in oxidative stress markers, amino acid utilization, and glycolytic activity, underscoring the acute biochemical dynamics triggered by epileptic episodes. Furthermore, the distinct metabolic profiles between focal and FBTC seizures emphasized the specific biochemical pathways involved in different seizure types, offering potential biomarkers for more precise diagnosis and personalized treatment.
The limitations of this study include the lack of longitudinal data, which restricts our ability to evaluate long-term metabolic alterations. Contextual factors, such as heterogeneity in medication regimens, unstandardized dietary intake, and the non-fasting state of post-seizure samples, introduce potential confounders that were not fully controlled. Additionally, the modest sample size limits statistical power and generalizability. Addressing these limitations in future research is crucial for a more comprehensive and nuanced understanding of the metabolic dynamics associated with epilepsy.
These findings offer valuable insights into the metabolic underpinnings of epilepsy, laying the groundwork for targeted therapies. Future research should focus on expanding cohort diversity and size, integrating multi-omics approaches, and systematically assessing the impact of antiepileptic therapies to elucidate the complex metabolic pathways underpinning epilepsy. These efforts will be pivotal in advancing the precision and effectiveness of therapeutic interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by Argentine Agency for Scientific and Technological Promotion and CONICET. MA and LP are members of the Scientific and Technological Researcher Career of CONICET, Argentina. JCA is a neurology staff member at the Italian Hospital of Buenos Aires, Argentina.
Author contributions
J.C.A. recruited subjects from the Epilepsy Section at the Italian Hospital of Buenos Aires, assessed inclusion criteria, and collected serum samples. L.P. and M.A. conducted NMR experiments, performed data analysis, and carried out the statistical evaluation. M.A. drafted the manuscript. J.C.A. and L.P. made substantial revisions to the manuscript and contributed to the literature review and references. M.A. supervised the overall project.
Data availability
Data is provided within the manuscript or supplementary information files. NMR spectra datasets generated during this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest regarding the research described in this paper.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juan C. Avalos and Leonardo Pellizza contributed equally to this work.
References
- 1.Behr, C., Goltzene, M. A., Kosmalski, G., Hirsch, E. & Ryvlin, P. Epidemiology of epilepsy. Rev. Neurol.172, 27–36. 10.1016/j.neurol.2015.11.003 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Devinsky, O. et al. Epilepsy. Nat. Reviews Disease Primers4, 18024, doi:10.1038/nrdp.2018.24 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Kwan, P. & Brodie, M. J. Early identification of refractory epilepsy. N. Engl. J. Med.342, 314–319. 10.1056/NEJM200002033420503 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Gesche, J. & Beier, C. P. Drug resistance in idiopathic generalized epilepsies: evidence and concepts. Epilepsia63, 3007–3019. 10.1111/epi.17410 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auvin, S. et al. Revisiting the concept of drug-resistant epilepsy: A TASK1 report of the ILAE/AES joint translational task force. Epilepsia64, 2891–2908. 10.1111/epi.17751 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong, D. et al. Multi-omics integration and epilepsy: towards a better Understanding of biological mechanisms. Prog. Neurobiol.227, 102480. 10.1016/j.pneurobio.2023.102480 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Boguszewicz, L. et al. NMR-based metabolomics in pediatric drug resistant epilepsy - preliminary results. Sci. Rep.9, 15035. 10.1038/s41598-019-51337-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murgia, F. et al. Metabolomics as a tool for the characterization of Drug-Resistant epilepsy. Front. Neurol.8, 459. 10.3389/fneur.2017.00459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emwas, A. H. et al. NMR spectroscopy for metabolomics research. Metabolites910.3390/metabo9070123 (2019). [DOI] [PMC free article] [PubMed]
- 10.Nagana Gowda, G. A., Gowda, Y. N. & Raftery, D. Expanding the limits of human blood metabolite quantitation using NMR spectroscopy. Anal. Chem.87, 706–715. 10.1021/ac503651e (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyamundanda, G., Gormley, I. C., Fan, Y., Gallagher, W. M. & Brennan, L. MetSizeR: selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinform.14, 338. 10.1186/1471-2105-14-338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashrafi, M. R. et al. A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia48, 1750–1755. 10.1111/j.1528-1167.2007.01143.x (2007). [DOI] [PubMed] [Google Scholar]
- 13.Aguiar, C. C. et al. Oxidative stress and epilepsy: literature review. Oxidative medicine and cellular longevity 795259, (2012). 10.1155/2012/795259 (2012). [DOI] [PMC free article] [PubMed]
- 14.Jarrett, S. G., Liang, L. P., Hellier, J. L., Staley, K. J. & Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis.30, 130–138. 10.1016/j.nbd.2007.12.009 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, S. J. & Yu, B. C. Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J. Bioenerg. Biomembr.42, 457–459. 10.1007/s10863-010-9317-4 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Mann, G., Mora, S., Madu, G. & Adegoke, O. A. J. Branched-chain amino acids: catabolism in skeletal muscle and implications for muscle and Whole-body metabolism. Front. Physiol.12, 702826. 10.3389/fphys.2021.702826 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, W. et al. Altered plasma glutamate and glutamine levels in patients with drug-resistant and drug-responsive symptomatic focal epilepsy. Neurosciences26, 315–322. 10.17712/nsj.2021.4.20210041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vettore, L. A., Westbrook, R. L. & Tennant, D. A. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids. 53, 1779–1788. 10.1007/s00726-021-03051-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muoio, D. M. et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabol.15, 764–777. 10.1016/j.cmet.2012.04.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scafidi, S., Racz, J., Hazelton, J., McKenna, M. C. & Fiskum, G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev. Neurosci.32, 480–487. 10.1159/000323178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hota, K. B., Hota, S. K., Chaurasia, O. P. & Singh, S. B. Acetyl-L-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus22, 723–736. 10.1002/hipo.20934 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Wang, L., Chen, P. & Xiao, W. beta-hydroxybutyrate as an Anti-Aging metabolite. Nutrients1310.3390/nu13103420 (2021). [DOI] [PMC free article] [PubMed]
- 23.Park, J. et al. Bioactive lipids and their derivatives in biomedical applications. Biomolecules Ther.29, 465–482. 10.4062/biomolther.2021.107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald, T., Puchowicz, M. & Borges, K. Impairments in oxidative glucose metabolism in epilepsy and metabolic treatments thereof. Front. Cell. Neurosci.12, 274. 10.3389/fncel.2018.00274 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunduz, M., Unal, O., Kavurt, S., Turk, E. & Mungan, N. O. Clinical findings and effect of sodium hydrogen carbonate in patients with glutathione synthetase deficiency. J. Pediatr. Endocrinol. Metabolism: JPEM. 29, 481–485. 10.1515/jpem-2015-0308 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Stewart, G. W. Pyroglutamate acidosis 2023. A review of 100 cases. Clin. Med.24, 100030. 10.1016/j.clinme.2024.100030 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gall, W. E. et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PloS One. 5, e10883. 10.1371/journal.pone.0010883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felger, J. C. et al. Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain. Behav. Immun.31, 153–160. 10.1016/j.bbi.2012.10.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutterer, M. et al. Epileptic activity increases cerebral amino acid transport assessed by 18F-Fluoroethyl-l-Tyrosine amino acid PET: A potential brain tumor mimic. J. Nuclear Medicine: Official Publication Soc. Nuclear Med.58, 129–137. 10.2967/jnumed.116.176610 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Godoi, A. B. et al. Circulating Metabolites as Biomarkers of Disease in Patients with Mesial Temporal Lobe Epilepsy. Metabolites 12, (2022). 10.3390/metabo12050446 [DOI] [PMC free article] [PubMed]
- 31.Hohn, A. et al. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol.11, 482–501. 10.1016/j.redox.2016.12.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling, Z. N. et al. Amino acid metabolism in health and disease. Signal. Transduct. Target. Therapy. 810.1038/s41392-023-01569-3 (2023). [DOI] [PMC free article] [PubMed]
- 33.Irwin, C. et al. Contribution towards a metabolite profile of the detoxification of benzoic acid through Glycine conjugation: an intervention study. PloS One. 11, e0167309. 10.1371/journal.pone.0167309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonilla, D. A. et al. Metabolic basis of creatine in health and disease: A Bioinformatics-Assisted review. Nutrients1310.3390/nu13041238 (2021). [DOI] [PMC free article] [PubMed]
- 35.Tang, Q., Tan, P., Ma, N. & Ma, X. Physiological functions of threonine in animals: beyond nutrition metabolism. Nutrients1310.3390/nu13082592 (2021). [DOI] [PMC free article] [PubMed]
- 36.Duan, Y. et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids. 48, 41–51. 10.1007/s00726-015-2067-1 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Mutafova-Yambolieva, V. N. & Durnin, L. The purinergic neurotransmitter revisited: a single substance or multiple players? Pharmacol. Ther.144, 162–191. 10.1016/j.pharmthera.2014.05.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files. NMR spectra datasets generated during this study are available from the corresponding author upon reasonable request.