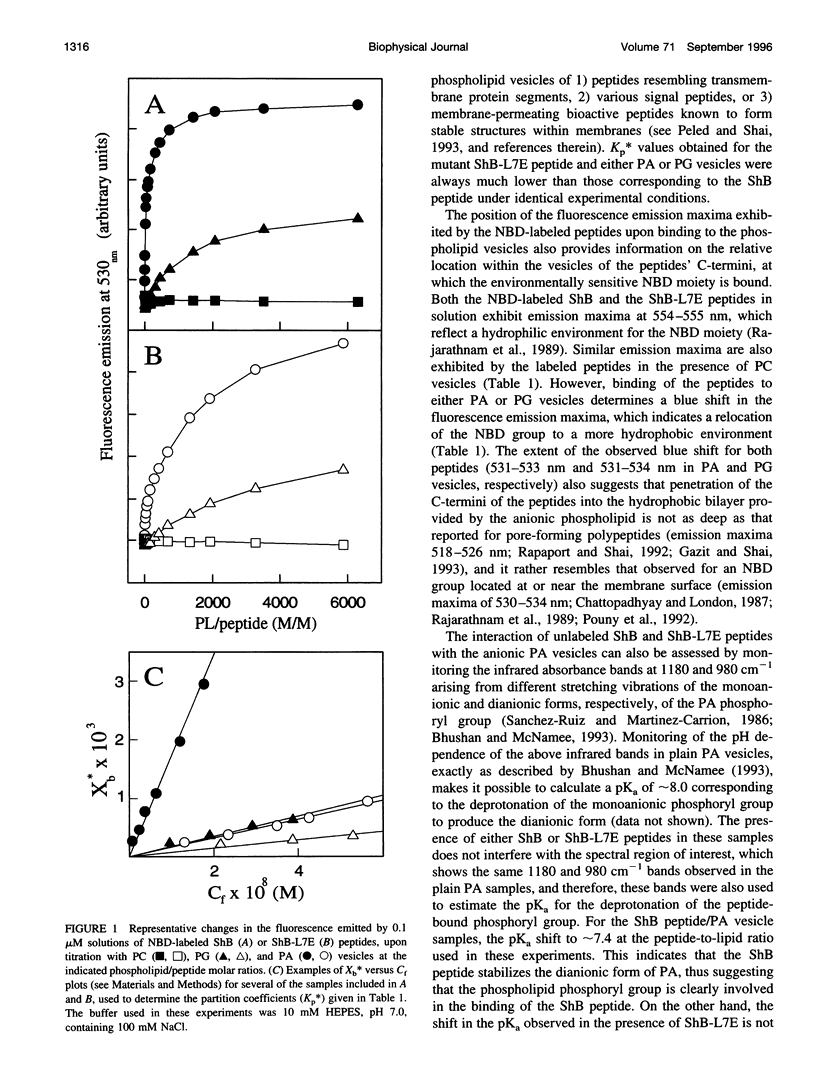
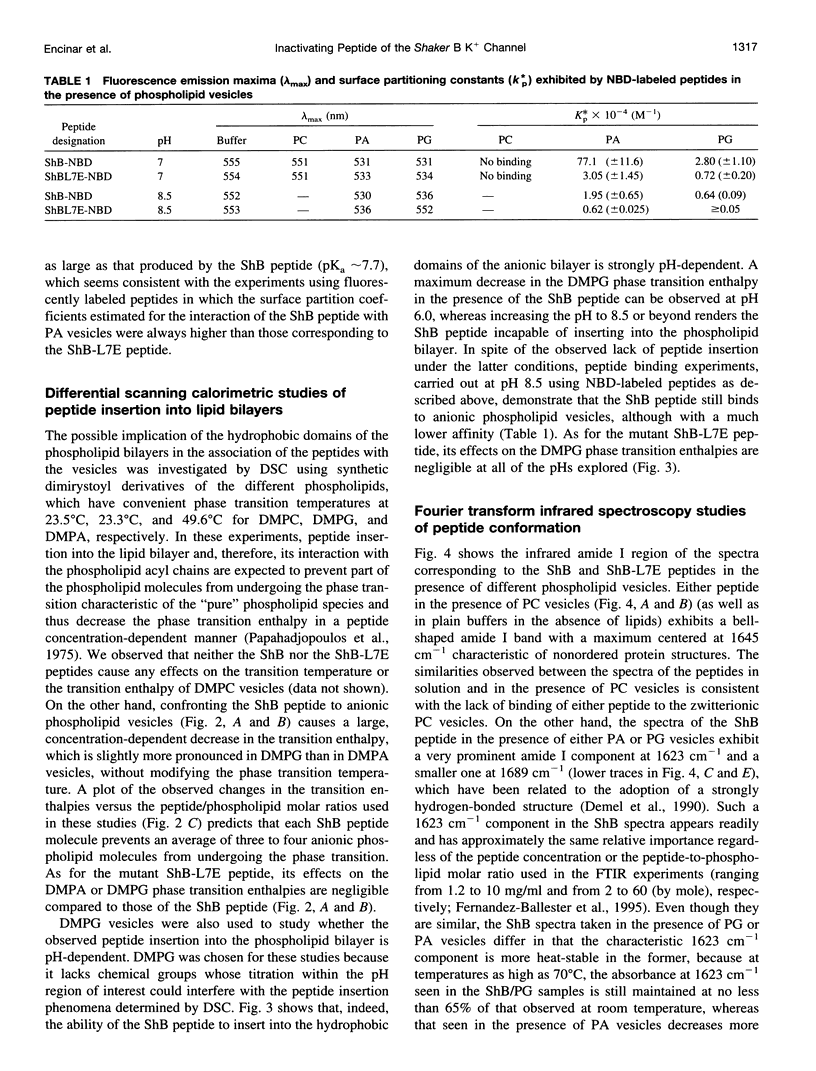
Abstract
Studies of rapid (N-type) inactivation induced by different synthetic inactivating peptides in several voltage-dependent cation channels have concluded that the channel inactivation "entrance" (or "receptor" site for the inactivating peptide) consists of a hydrophobic vestibule within the internal mouth of the channel, separated from the cytoplasm by a region with a negative surface potential. These protein domains are conformed from alternative sequences in the different channels and thus are relatively unrestricted in terms of primary structure. We are reporting here on the interaction between the inactivating peptide of the Shaker B K+ channel (ShB peptide) or the noninactivating ShB-L7E mutant with anionic phospholipid vesicles, a model target that, as the channel's inactivation "entrance," contains a hydrophobic domain (the vesicle bilayer) separated from the aqueous media by a negatively charged vesicle surface. When challenged by the anionic phospholipid vesicles, the inactivating ShB peptide 1) binds to the vesicle surface with a relatively high affinity, 2) readily adopts a strongly hydrogen-bonded beta-structure, likely an intramolecular beta "hairpin," and 3) becomes inserted into the hydrophobic bilayer by its folded N-terminal portion, leaving its positively charged C-terminal end exposed to the extravesicular aqueous medium. Similar experiments carried out with the noninactivating, L7E-ShB mutant peptide show that this peptide 1) binds also to the anionic vesicles, although with a lower affinity than does the ShB peptide, 2) adopts only occasionally the characteristic beta-structure, and 3) has completely lost the ability to traverse the anionic interphase at the vesicle surface and to insert into the hydrophobic vesicle bilayer. Because the negatively charged surface and the hydrophobic domains in the model target may partly imitate those conformed at the inactivation "entrance" of the channel proteins, we propose that channel inactivation likely includes molecular events similar to those observed in the interaction of the ShB peptide with the phospholipid vesicles, i.e., binding of the peptide to the region of negative surface potential, folding of the bound peptide as a beta-structure, and its insertion into the channel's hydrophobic vestibule. Likewise, we relate the lack of channel inactivation seen with the mutant ShB-L7E peptide to the lack of ability shown by this peptide to cross through the anionic interphase and insert into the hydrophobic domains of the model vesicle target.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A., McNamee M. G. Correlation of phospholipid structure with functional effects on the nicotinic acetylcholine receptor. A modulatory role for phosphatidic acid. Biophys J. 1993 Mar;64(3):716–723. doi: 10.1016/S0006-3495(93)81431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J., Fernandez-Ballester G., Fernandez A. M., Laynez J. L., Arrondo J. L., Ferragut J. A., Gonzalez-Ros J. M. Protein structural effects of agonist binding to the nicotinic acetylcholine receptor. FEBS Lett. 1992 Dec 14;314(2):171–175. doi: 10.1016/0014-5793(92)80967-l. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987 Jan 13;26(1):39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Goormaghtigh E., de Kruijff B. Lipid and peptide specificities in signal peptide--lipid interactions in model membranes. Biochim Biophys Acta. 1990 Aug 24;1027(2):155–162. doi: 10.1016/0005-2736(90)90079-4. [DOI] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991 Nov;7(5):743–753. doi: 10.1016/0896-6273(91)90277-7. [DOI] [PubMed] [Google Scholar]
- Dubinsky W. P., Mayorga-Wark O., Schultz S. G. A peptide from the Drosophila Shaker K+ channel inhibits a voltage-gated K+ channel in basolateral membranes of Necturus enterocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1770–1774. doi: 10.1073/pnas.89.5.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ballester G., Gavilanes F., Albar J. P., Criado M., Ferragut J. A., Gonzalez-Ros J. M. Adoption of beta structure by the inactivating "ball" peptide of the Shaker B potassium channel. Biophys J. 1995 Mar;68(3):858–865. doi: 10.1016/S0006-3495(95)80262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C. D., Chung S., Zagotta W. N., Aldrich R. W., Levitan I. B. A peptide derived from the Shaker B K+ channel produces short and long blocks of reconstituted Ca(2+)-dependent K+ channels. Neuron. 1992 Aug;9(2):229–236. doi: 10.1016/0896-6273(92)90162-7. [DOI] [PubMed] [Google Scholar]
- Gazit E., Shai Y. Structural and functional characterization of the alpha 5 segment of Bacillus thuringiensis delta-endotoxin. Biochemistry. 1993 Apr 6;32(13):3429–3436. doi: 10.1021/bi00064a029. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Goulding E., Siegelbaum S. A. Potassium channel inactivation peptide blocks cyclic nucleotide-gated channels by binding to the conserved pore domain. Neuron. 1994 Mar;12(3):655–662. doi: 10.1016/0896-6273(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Labarca P., Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995 Mar;268(3 Pt 1):C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- Murrell-Lagnado R. D., Aldrich R. W. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J Gen Physiol. 1993 Dec;102(6):949–975. doi: 10.1085/jgp.102.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Moscarello M., Eylar E. H., Isac T. Effects of proteins on thermotropic phase transitions of phospholipid membranes. Biochim Biophys Acta. 1975 Sep 2;401(3):317–335. doi: 10.1016/0005-2736(75)90233-3. [DOI] [PubMed] [Google Scholar]
- Patton D. E., West J. W., Catterall W. A., Goldin A. L. A peptide segment critical for sodium channel inactivation functions as an inactivation gate in a potassium channel. Neuron. 1993 Nov;11(5):967–974. doi: 10.1016/0896-6273(93)90125-b. [DOI] [PubMed] [Google Scholar]
- Peled H., Shai Y. Membrane interaction and self-assembly within phospholipid membranes of synthetic segments corresponding to the H-5 region of the shaker K+ channel. Biochemistry. 1993 Aug 10;32(31):7879–7885. doi: 10.1021/bi00082a007. [DOI] [PubMed] [Google Scholar]
- Pouny Y., Rapaport D., Mor A., Nicolas P., Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry. 1992 Dec 15;31(49):12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- Rajarathnam K., Hochman J., Schindler M., Ferguson-Miller S. Synthesis, location, and lateral mobility of fluorescently labeled ubiquinone 10 in mitochondrial and artificial membranes. Biochemistry. 1989 Apr 18;28(8):3168–3176. doi: 10.1021/bi00434a009. [DOI] [PubMed] [Google Scholar]
- Rapaport D., Shai Y. Aggregation and organization of pardaxin in phospholipid membranes. A fluorescence energy transfer study. J Biol Chem. 1992 Apr 5;267(10):6502–6509. [PubMed] [Google Scholar]
- Ruppersberg J. P., Frank R., Pongs O., Stocker M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 1991 Oct 17;353(6345):657–660. doi: 10.1038/353657a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruiz J. M., Martinez-Carrion M. Ionization state of the coenzyme 5'-phosphate ester in cytosolic aspartate aminotransferase. A Fourier transform infrared spectroscopic study. Biochemistry. 1986 May 20;25(10):2915–2920. doi: 10.1021/bi00358a027. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993 Jan 19;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Toro L., Ottolia M., Stefani E., Latorre R. Structural determinants in the interaction of Shaker inactivating peptide and a Ca(2+)-activated K+ channel. Biochemistry. 1994 Jun 14;33(23):7220–7228. doi: 10.1021/bi00189a026. [DOI] [PubMed] [Google Scholar]
- Toro L., Stefani E., Latorre R. Internal blockade of a Ca(2+)-activated K+ channel by Shaker B inactivating "ball" peptide. Neuron. 1992 Aug;9(2):237–245. doi: 10.1016/0896-6273(92)90163-8. [DOI] [PubMed] [Google Scholar]
- Villar M. T., Artigues A., Ferragut J. A., Gonzalez-Ros J. M. Phospholipase A2 hydrolysis of membrane phospholipids causes structural alteration of the nicotinic acetylcholine receptor. Biochim Biophys Acta. 1988 Feb 8;938(1):35–43. doi: 10.1016/0005-2736(88)90119-8. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Hodges R. S., McElhaney R. N. FTIR spectroscopic studies of the conformation and amide hydrogen exchange of a peptide model of the hydrophobic transmembrane alpha-helices of membrane proteins. Biochemistry. 1992 Nov 24;31(46):11572–11578. doi: 10.1021/bi00161a041. [DOI] [PubMed] [Google Scholar]


