Abstract
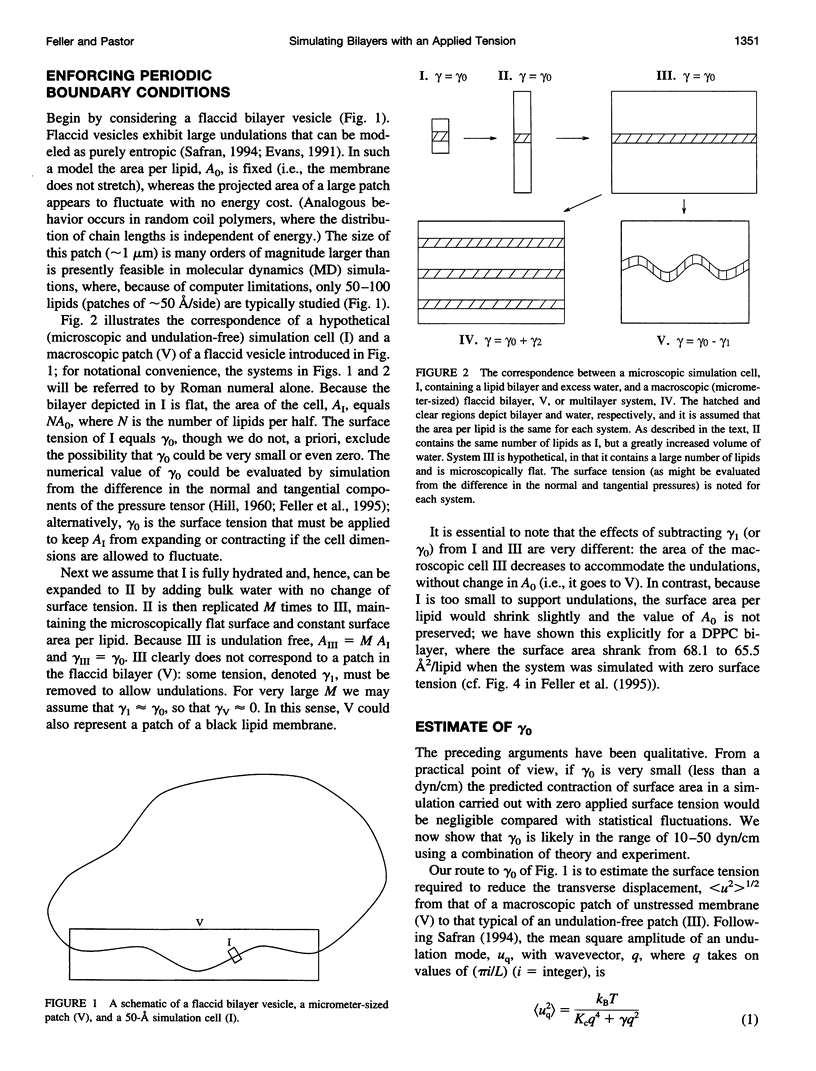
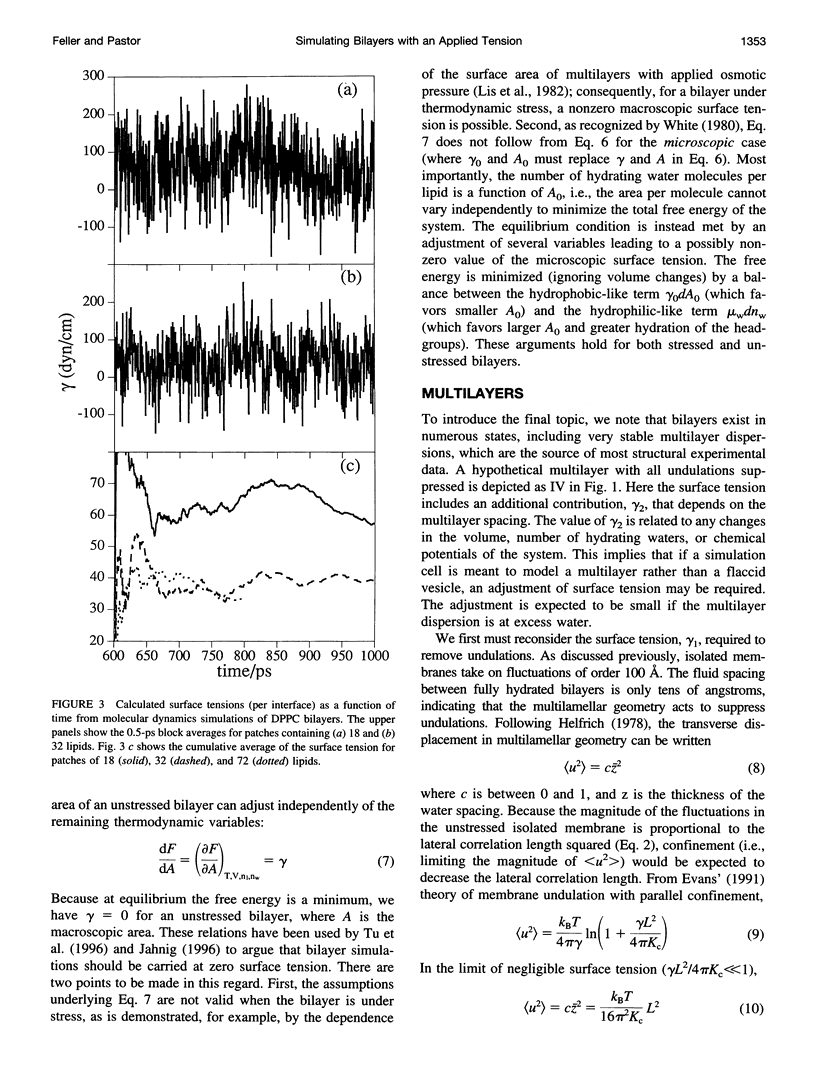
As sketched in Fig. 1, a current molecular dynamics computer simulation of a lipid bilayer fails to capture significant features of the macroscopic system, including long wavelength undulations. Such fluctuations are intrinsically connected to the value of the macroscopic (or thermodynamic) surface tension (cf. Eqs. 1 and 9; for a related treatment, see Brochard et al., 1975, 1976). Consequently, the surface tension that might be evaluated in an MD simulation should not be expected to equal the surface tension obtained from macroscopic measurements. Put another way, the largest of the three simulations presented here contained over 16,000 atoms and required substantial computer time to complete, but modeled a system of only 36 lipids per side. From this perspective it is not surprising that the system is not at the thermodynamic limit. An important practical consequence of this effect is that simulations with fluctuating area should be carried out with a nonzero applied surface tension (gamma 0 of Fig. 2) even when the macroscopic tension is zero, or close to zero. Computer simulations at fixed surface area, which can explicitly determine pressure anisotropy at the molecular level, should ultimately lend insight into the value of gamma 0, including its dependence on lipid composition and other membrane components. As we have noted and will describe further in separate publications (Feller et al., 1996; Feller et al., manuscript in preparation), surface tensions obtained from simulations can be distorted by inadequate initial conditions and convergence, and are sensitive to potential energy functions, force truncation methods, and system size; it is not difficult, in fact, to tune terms in the potential energy function so as to yield surface tensions close to zero. This is why parameters should be tested extensively on simpler systems, for example, monolayers. The estimates of gamma 0 that we have presented here should be regarded as qualitative, and primarily underscore the assertion that the surface tension of a microscopically flat, simulation-sized patch is significantly greater than zero. As the simulation cell length increases, the surface tension that would be evaluated (or should be applied) decreases; in the limit of micrometer-sized simulation cells, gamma would approach zero or its appropriate thermodynamic value. The theories presented here also imply that the estimation of bilayer surface tension from monolayer data should take the degree of flatness into account. These conclusions are independent of the precise values of parameters such as bending constants. In conclusion, from the simulator's perspective, the question "What is the surface tension of a bilayer?" is better phrased as "What is the value of the applied surface tension necessary to simulate a particular experimental system with a given number of lipids?". As we have shown, the answer to the second question varies, but it should not be assumed a priori to equal zero.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiu S. W., Clark M., Balaji V., Subramaniam S., Scott H. L., Jakobsson E. Incorporation of surface tension into molecular dynamics simulation of an interface: a fluid phase lipid bilayer membrane. Biophys J. 1995 Oct;69(4):1230–1245. doi: 10.1016/S0006-3495(95)80005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990 Apr 23;64(17):2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Jähnig F. What is the surface tension of a lipid bilayer membrane? Biophys J. 1996 Sep;71(3):1348–1349. doi: 10.1016/S0006-3495(96)79336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A. Lipid monolayer states and their relationships to bilayers. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Zhang R., Tristram-Nagle S., Sun W., Petrache H. I., Suter R. M. X-ray structure determination of fully hydrated L alpha phase dipalmitoylphosphatidylcholine bilayers. Biophys J. 1996 Mar;70(3):1419–1431. doi: 10.1016/S0006-3495(96)79701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Tanford C. Hydrostatic pressure in small phospholipid vesicles. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3318–3319. doi: 10.1073/pnas.76.7.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu K., Tobias D. J., Blasie J. K., Klein M. L. Molecular dynamics investigation of the structure of a fully hydrated gel-phase dipalmitoylphosphatidylcholine bilayer. Biophys J. 1996 Feb;70(2):595–608. doi: 10.1016/S0006-3495(96)79623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Small phospholipid vesicles: internal pressure, surface tension, and surface free energy. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4048–4050. doi: 10.1073/pnas.77.7.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure. Biophys J. 1992 Feb;61(2):434–447. doi: 10.1016/S0006-3495(92)81849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


