Abstract
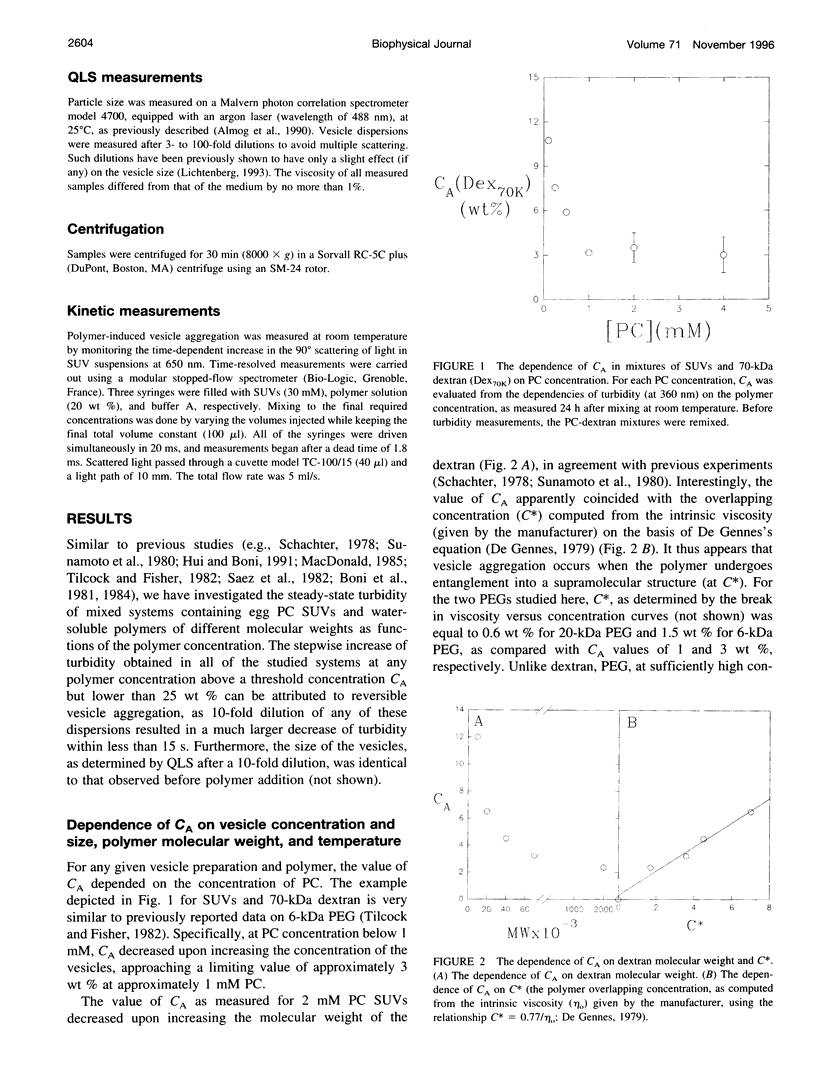
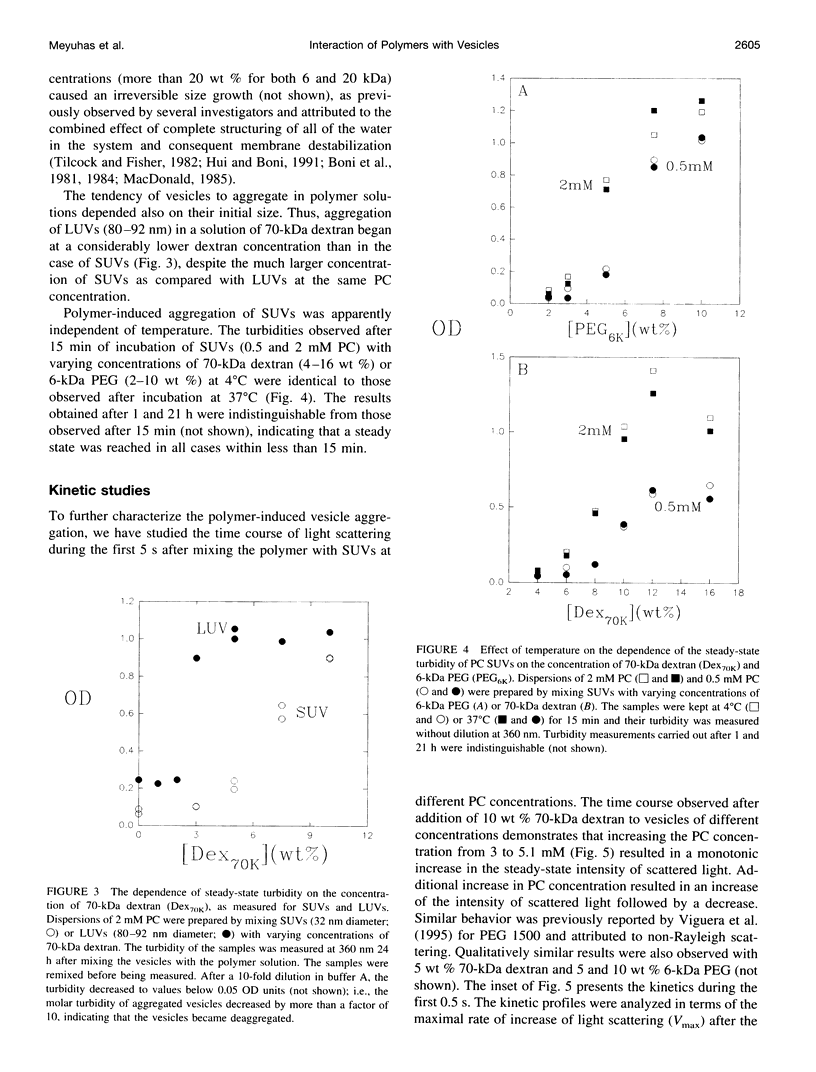
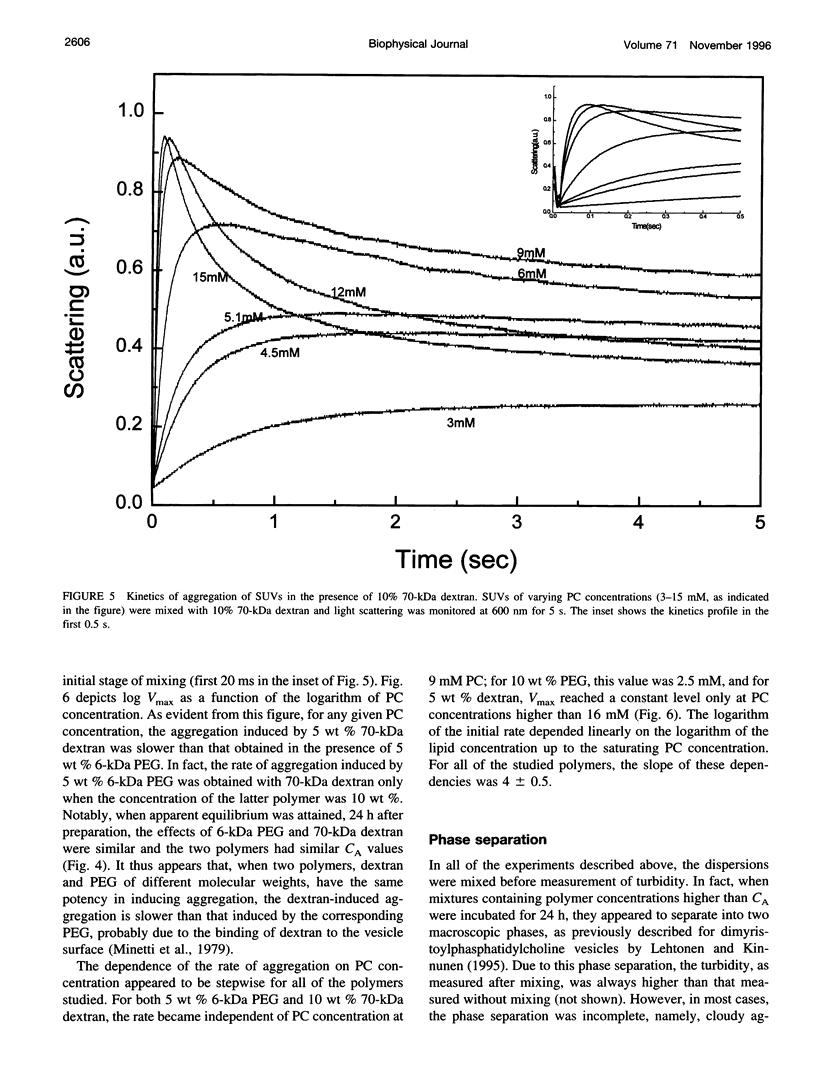
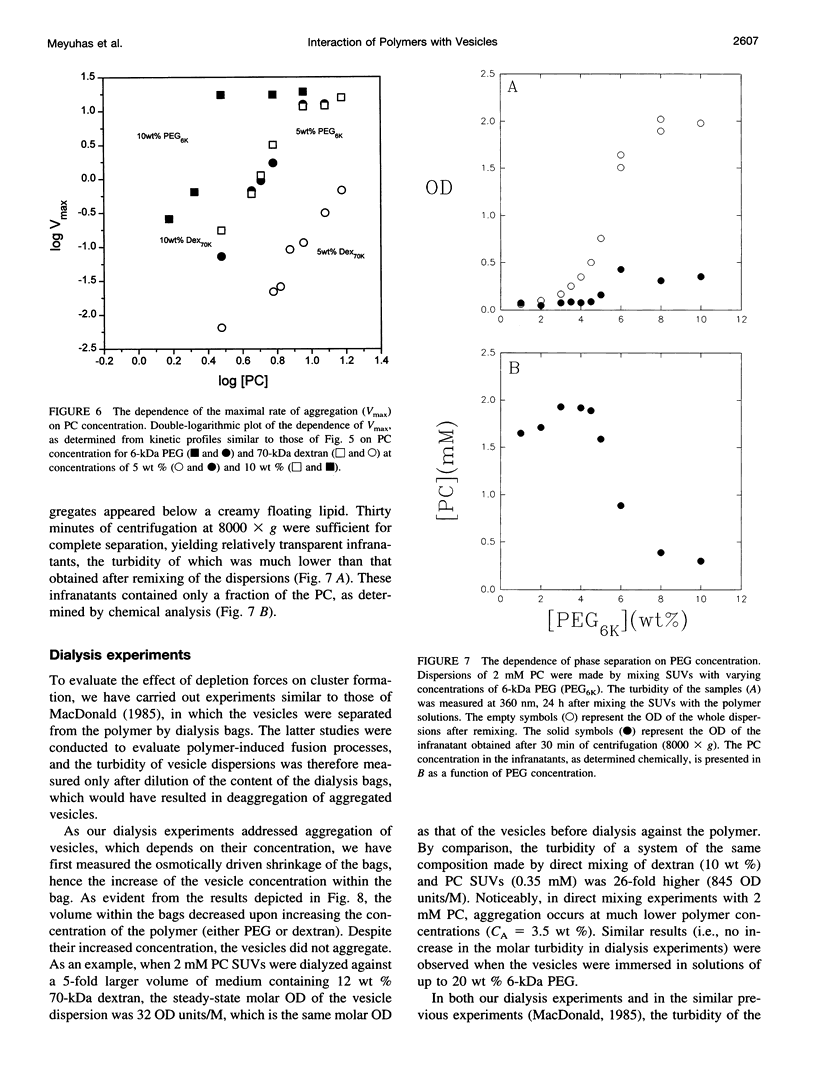
Water-soluble polymers such as dextran and polyethylene glycol are known to induce aggregation and size growth of phospholipid vesicles. The present study addresses the dependence of these processes on vesicle size and concentration, polymer molecular weight, temperature, and compartmentalization of the vesicles and polymers, using static and dynamic light scattering. Increasing the molecular weight of the polymers resulted in a reduction of the concentration of polymer needed for induction of aggregation of small unilamellar vesicles. The aggregation was fully reversible (by dilution), within a few seconds, up to a polymer concentration of at least 20 wt %. At relatively low phosphatidylcholine (PC) concentrations (up to approximately 1 mM), increasing the PC concentration resulted in faster kinetics of aggregation and reduced the threshold concentration of polymer required for rapid aggregation (CA). At higher PC concentrations, CA was only slightly dependent on the concentration of PC and was approximately equal to the overlapping concentration of the polymer (C*). The extent of aggregation was similar at 37 and 4 degrees C. Aggregation of large unilamellar vesicles required a lower polymer concentration, probably because aggregation occurs in a secondary minimum (without surface contact). In contrast to experiments in which the polymers were added directly to the vesicles, dialysis of the vesicles against polymer-containing solutions did not induce aggregation. Based on this result, it appears that exclusion of polymer from the hydration sphere of vesicles and the consequent depletion of polymer molecules from clusters of aggregated vesicles play the central role in the induction of reversible vesicle aggregation. The results of all the other experiments are consistent with this conclusion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog S., Litman B. J., Wimley W., Cohen J., Wachtel E. J., Barenholz Y., Ben-Shaul A., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990 May 15;29(19):4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Arnold K., Zschoernig O., Barthel D., Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990 Mar;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Hah J. S., Hui S. W., Mukherjee P., Ho J. T., Jung C. Y. Aggregation and fusion of unilamellar vesicles by poly(ethylene glycol). Biochim Biophys Acta. 1984 Sep 5;775(3):409–418. doi: 10.1016/0005-2736(84)90198-6. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Stewart T. P., Alderfer J. L., Hui S. W. Lipid-polyethylene glycol interactions: I. Induction of fusion between liposomes. J Membr Biol. 1981;62(1-2):65–70. doi: 10.1007/BF01870200. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., Massenburg D., Yates J., Lentz B. R. Poly(ethylene glycol)-induced lipid mixing but not fusion between synthetic phosphatidylcholine large unilamellar vesicles. Biochemistry. 1991 Apr 30;30(17):4193–4200. doi: 10.1021/bi00231a013. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., McIntosh T. J., Lentz B. R. Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry. 1992 Mar 17;31(10):2653–2661. doi: 10.1021/bi00125a004. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Induction of mammalian somatic cell hybridization by polyethylene glycol. Methods Cell Biol. 1977;15:325–338. doi: 10.1016/s0091-679x(08)60223-x. [DOI] [PubMed] [Google Scholar]
- Day E. P., Kwok A. Y., Hark S. K., Ho J. T., Vail W. J., Bentz J., Nir S. Reversibility of sodium-induced aggregation of sonicated phosphatidylserine vesicles. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4026–4029. doi: 10.1073/pnas.77.7.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of mixed phosphatidylcholine/phosphatidylserine lipid vesicles in glucose polymer (dextran) solutions. Biophys J. 1984 Apr;45(4):715–720. doi: 10.1016/S0006-3495(84)84213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Knutton S. Studies of membrane fusion. III. Fusion of erythrocytes with polyethylene glycol. J Cell Sci. 1979 Apr;36:61–72. doi: 10.1242/jcs.36.1.61. [DOI] [PubMed] [Google Scholar]
- Krähling H. Discrimination of two fusogenic properties of aqueous polyethylene glycol solutions. Z Naturforsch C. 1981 Jul-Aug;36(7-8):593–596. doi: 10.1515/znc-1981-7-814. [DOI] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Changes in the lipid dynamics of liposomal membranes induced by poly(ethylene glycol): free volume alterations revealed by inter- and intramolecular excimer-forming phospholipid analogs. Biophys J. 1994 Jun;66(6):1981–1990. doi: 10.1016/S0006-3495(94)80991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Poly(ethylene glycol)-induced and temperature-dependent phase separation in fluid binary phospholipid membranes. Biophys J. 1995 Feb;68(2):525–535. doi: 10.1016/S0006-3495(95)80214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Carpenter T. J., Alford D. R. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987 Aug 25;26(17):5389–5397. doi: 10.1021/bi00391a026. [DOI] [PubMed] [Google Scholar]
- MacDonald R. I. Membrane fusion due to dehydration by polyethylene glycol, dextran, or sucrose. Biochemistry. 1985 Jul 16;24(15):4058–4066. doi: 10.1021/bi00336a039. [DOI] [PubMed] [Google Scholar]
- Massenburg D., Lentz B. R. Poly(ethylene glycol)-induced fusion and rupture of dipalmitoylphosphatidylcholine large, unilamellar extruded vesicles. Biochemistry. 1993 Sep 7;32(35):9172–9180. doi: 10.1021/bi00086a024. [DOI] [PubMed] [Google Scholar]
- Meyuhas D., Lichtenberg D. Effect of water-soluble polymers on the state of aggregation, vesicle size, and phase transformations in mixtures of phosphatidylcholine and sodium cholate. Biophys J. 1996 Nov;71(5):2613–2622. doi: 10.1016/S0006-3495(96)79453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti M., Aducci P., Viti V. Interaction of neutral polysaccharides with phosphatidylcholine multilamellar liposomes. Phase transitions studied by the binding of fluorescein-conjugated dextrans. Biochemistry. 1979 Jun 12;18(12):2541–2548. doi: 10.1021/bi00579a017. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., Chan S. I. The effects of the thermal prephase transition and salts on the coagulation and flocculation of phosphatidylcholine bilayer vesicles. Biochim Biophys Acta. 1978 May 4;509(1):111–128. doi: 10.1016/0005-2736(78)90012-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Roos D. S., Davidson R. L., Karnovsky M. J. Membrane alterations and other morphological features associated with polyethylene glycol-induced cell fusion. J Cell Sci. 1979 Dec;40:63–75. doi: 10.1242/jcs.40.1.63. [DOI] [PubMed] [Google Scholar]
- Schachter D. Aggregation of liposomes by dextrans of high molecular weight. Biochem Biophys Res Commun. 1978 Oct 30;84(4):840–844. doi: 10.1016/0006-291x(78)91660-1. [DOI] [PubMed] [Google Scholar]
- Sunamoto J., Iwamoto K., Kondo H., Shinkai S. Liposomal membranes. VI. Polysaccharide-induced aggregation of multilamellar liposomes of egg lecithin. J Biochem. 1980 Nov;88(5):1219–1226. doi: 10.1093/oxfordjournals.jbchem.a133089. [DOI] [PubMed] [Google Scholar]
- Sáez R., Alonso A., Villena A., Goñi F. M. Detergent-like properties of polyethyleneglycols in relation to model membranes. FEBS Lett. 1982 Jan 25;137(2):323–326. doi: 10.1016/0014-5793(82)80376-1. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Fisher D. The interaction of phospholipid membranes with poly(ethylene glycol). Vesicle aggregation and lipid exchange. Biochim Biophys Acta. 1982 Jun 14;688(2):645–652. doi: 10.1016/0005-2736(82)90375-3. [DOI] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Lumley-Sapanski K., Jacobson K. A. Studies on the mechanism of polyethylene glycol-mediated cell fusion using fluorescent membrane and cytoplasmic probes. J Cell Biol. 1983 Jan;96(1):151–159. doi: 10.1083/jcb.96.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M., Thompson T. E. Aggregation of dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1982 Aug 17;21(17):4133–4139. doi: 10.1021/bi00260a033. [DOI] [PubMed] [Google Scholar]
- Wu J. R., Lentz B. R. Mechanism of poly(ethylene glycol)-induced lipid transfer between phosphatidylcholine large unilamellar vesicles: a fluorescent probe study. Biochemistry. 1991 Jul 9;30(27):6780–6787. doi: 10.1021/bi00241a022. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Ohnishi S., Ito T. Osmoelastic coupling in biological structures: decrease in membrane fluidity and osmophobic association of phospholipid vesicles in response to osmotic stress. Biochemistry. 1989 May 2;28(9):3710–3715. doi: 10.1021/bi00435a013. [DOI] [PubMed] [Google Scholar]


