Abstract
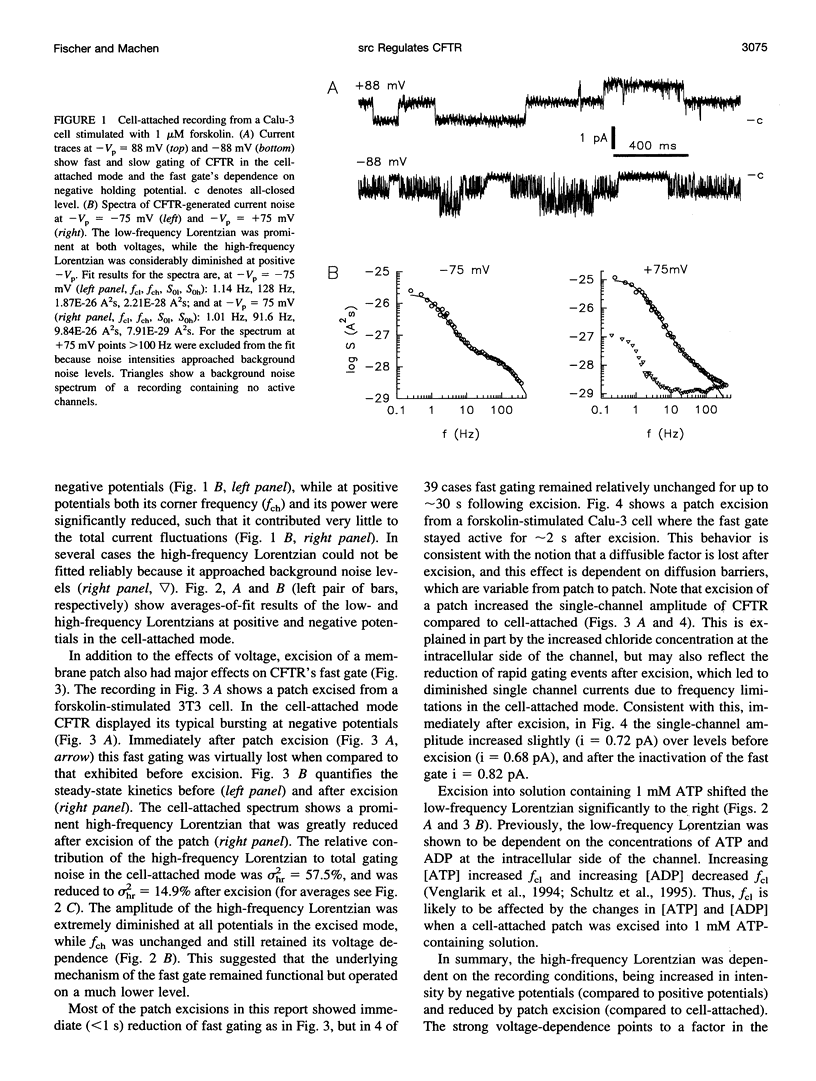
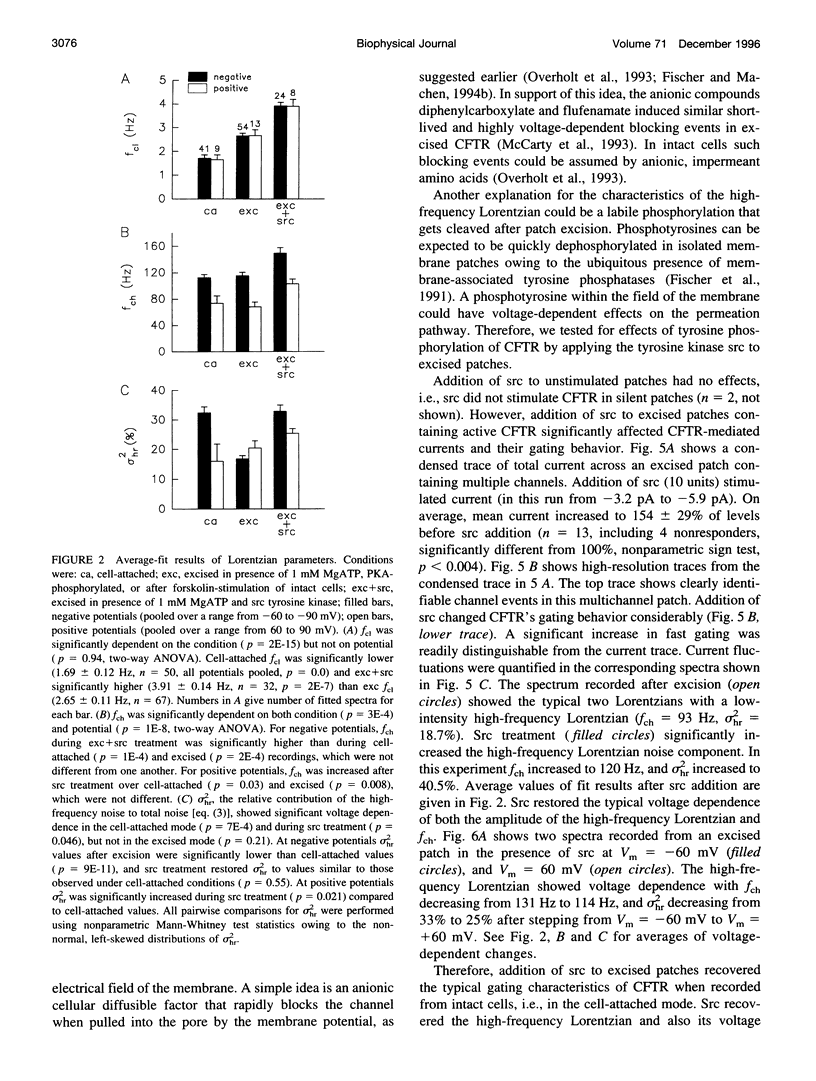
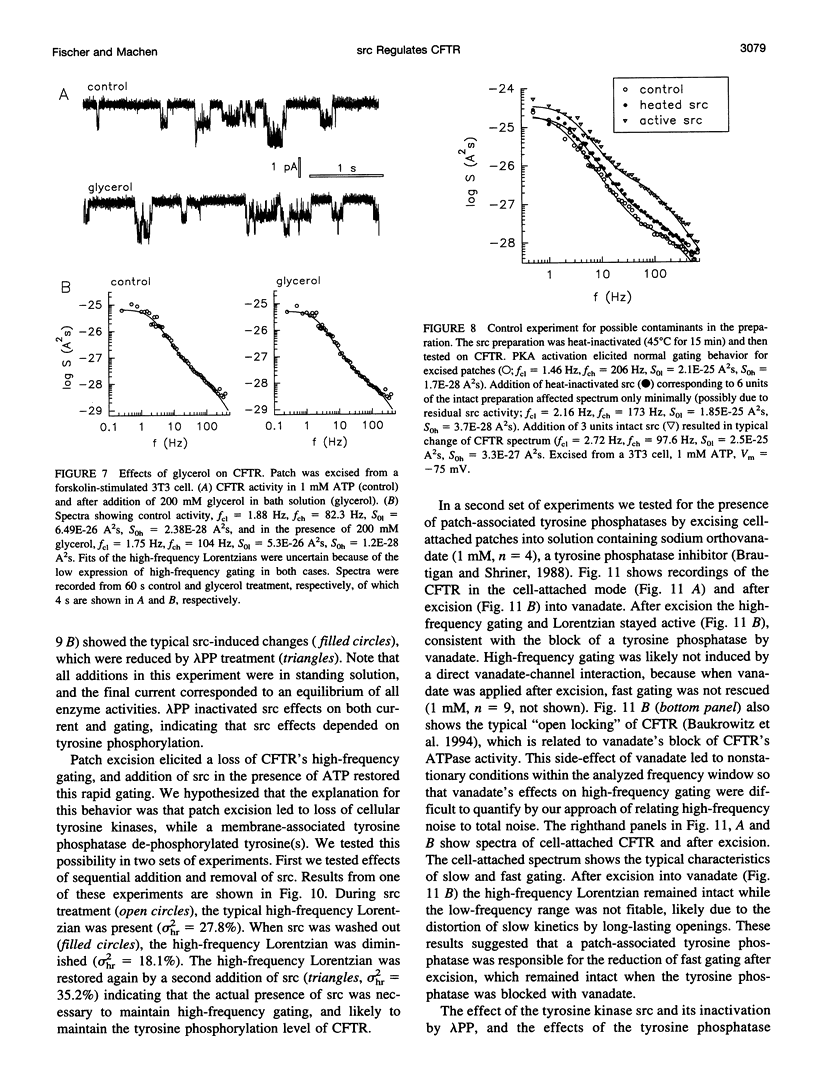
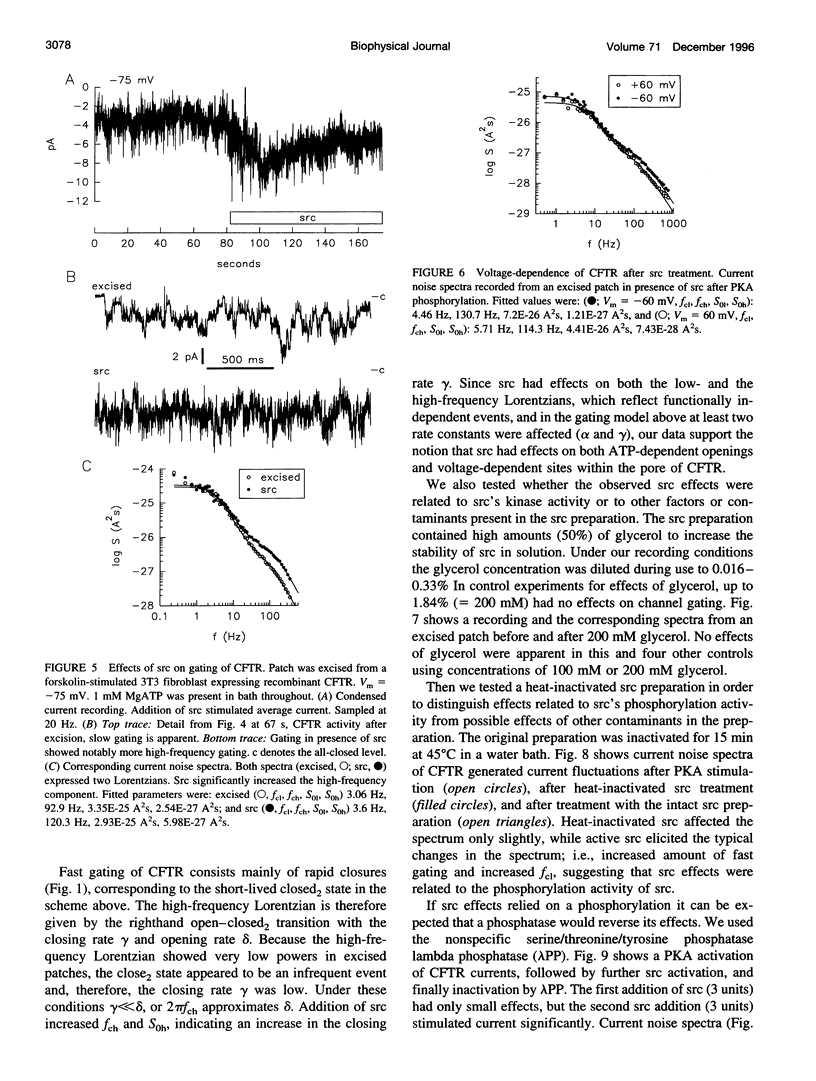
The role of the tyrosine kinase p60c-src on the gating of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel was investigated with the cell-attached and excised patch clamp technique in conjunction with current noise analysis of recordings containing multiple channels per patch. Spectra of CFTR-generated current noise contained a low-frequency and a high-frequency Lorentzian noise component. In the cell-attached mode, the high-frequency Lorentzian was significantly dependent on the membrane potential, while the low-frequency Lorentzian was unaffected. Excision of forskolin-stimulated patches into ATP-containing solution significantly reduced the amplitude of the voltage-dependent high-frequency Lorentzian. Addition of the tyrosine kinase p60c-src to excised, active, CFTR-containing membrane patches increased mean currents by 54%, increased the corner frequency of the low-frequency Lorentzian, and recovered the high-frequency Lorentzian and its characteristics. Treatment with lambda-phosphatase inactivated src-induced currents and changes in gating. When active patches were excised under conditions in which patch-associated tyrosine phosphatases were blocked with sodium vanadate, the high-frequency gating remained relatively unchanged. The results suggest that CFTR's open probability and its voltage-dependent fast gate are dependent on tyrosine phosphorylation, and that membrane-associated tyrosine phosphatases are responsible for inactivation of the fast gate after patch excision.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Kaufmann C., Cook T. A., Archdeacon P. Amino acid residues lining the chloride channel of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994 May 27;269(21):14865–14868. [PubMed] [Google Scholar]
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Hwang T. C., Nairn A. C., Gadsby D. C. Coupling of CFTR Cl- channel gating to an ATP hydrolysis cycle. Neuron. 1994 Mar;12(3):473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Bischof G., Illek B., Reenstra W. W., Machen T. E. Role for tyrosine kinases in carbachol-regulated Ca entry into colonic epithelial cells. Am J Physiol. 1995 Jan;268(1 Pt 1):C154–C161. doi: 10.1152/ajpcell.1995.268.1.C154. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Shriner C. L. Methods to distinguish various types of protein phosphatase activity. Methods Enzymol. 1988;159:339–346. doi: 10.1016/0076-6879(88)59034-1. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991 Sep 6;66(5):1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Fischer H., Machen T. E. CFTR displays voltage dependence and two gating modes during stimulation. J Gen Physiol. 1994 Sep;104(3):541–566. doi: 10.1085/jgp.104.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Harris A., Coleman L., Greenwell J. R., Argent B. E. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989 Aug;257(2 Pt 1):C240–C251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- Gunderson K. L., Kopito R. R. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J Biol Chem. 1994 Jul 29;269(30):19349–19353. [PubMed] [Google Scholar]
- Haws C., Finkbeiner W. E., Widdicombe J. H., Wine J. J. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl- conductance. Am J Physiol. 1994 May;266(5 Pt 1):L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- Haws C., Krouse M. E., Xia Y., Gruenert D. C., Wine J. J. CFTR channels in immortalized human airway cells. Am J Physiol. 1992 Dec;263(6 Pt 1):L692–L707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. M., Toscas K., Villereal M. L. Inhibition of bradykinin- and thapsigargin-induced Ca2+ entry by tyrosine kinase inhibitors. J Biol Chem. 1993 May 15;268(14):9945–9948. [PubMed] [Google Scholar]
- Matsumoto P. S., Ohara A., Duchatelle P., Eaton D. C. Tyrosine kinase regulates epithelial sodium transport in A6 cells. Am J Physiol. 1993 Jan;264(1 Pt 1):C246–C250. doi: 10.1152/ajpcell.1993.264.1.C246. [DOI] [PubMed] [Google Scholar]
- McCarty N. A., McDonough S., Cohen B. N., Riordan J. R., Davidson N., Lester H. A. Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl- channel by two closely related arylaminobenzoates. J Gen Physiol. 1993 Jul;102(1):1–23. doi: 10.1085/jgp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt J. L., Hobert M. E., Harvey R. D. On the mechanism of rectification of the isoproterenol-activated chloride current in guinea-pig ventricular myocytes. J Gen Physiol. 1993 Nov;102(5):871–895. doi: 10.1085/jgp.102.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M. R., Cohn J. A., Bertuzzi G., Greengard P., Nairn A. C. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1992 Jun 25;267(18):12742–12752. [PubMed] [Google Scholar]
- Schultz B. D., Venglarik C. J., Bridges R. J., Frizzell R. A. Regulation of CFTR Cl- channel gating by ADP and ATP analogues. J Gen Physiol. 1995 Mar;105(3):329–361. doi: 10.1085/jgp.105.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995 Feb 9;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991 Aug 15;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tsui L. C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992 Nov;8(11):392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Venglarik C. J., Schultz B. D., Frizzell R. A., Bridges R. J. ATP alters current fluctuations of cystic fibrosis transmembrane conductance regulator: evidence for a three-state activation mechanism. J Gen Physiol. 1994 Jul;104(1):123–146. doi: 10.1085/jgp.104.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. F., Kaczmarek L. K. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993 Dec 2;366(6454):433–438. doi: 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]


