Abstract
Current assays used to detect Mycobacterium bovis infection lack accuracy, especially for recently infected animals, or are impractical for rapid field diagnostic applications. To overcome these limitations with serological assays, a synthetic peptide derived from early secretory antigenic target 6 (ESAT6-p) and a recombinant major secreted immunogenic protein (rMPB70) of M. bovis were used in an enzyme-linked immunosorbent assay (EIA), an immunochromatographic assay (ICGA), and a latex bead agglutination assay (LBAA). Sera from noninfected, M. bovis-infected, or M. avium subsp. paratuberculosis-infected (by natural and experimental routes) animals were evaluated. Receiver operating characteristic analysis comparing optical density values from the EIA with results of bacterial culture or skin test, the reference test, established suitable cutoff values for assessing sensitivity and specificity. The EIA and LBAA, respectively, had sensitivities of 98.6 and 94.8%, specificities of 98.5 and 92.6%, and kappa values of 0.97 and 0.88 with ESAT6-p. The EIA, ICGA, and LBAA, respectively, had sensitivities of 96.8, 83.0, and 86.7%, specificities of 90.1, 99.4, and 97.8%, and kappa values of 0.87, 0.85, and 0.83 with rMPB70. Examination of serial samples of sera collected from experimentally M. bovis-infected cattle and deer revealed that ESAT6-p-specific responses developed early after infection whereas responses to rMPB70 developed later in the course of disease. The advantage of the LBAA and ICGA as initial tests for multiple species is a rapid reaction obtained in 2 to 3 h by LBAA or 20 min by ICGA without species-specific secondary antibodies under field conditions, thus allowing immediate segregation of suspect animals for further testing before culling.
Bovine tuberculosis (bTB) is caused by Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex. bTB is one of the important bovine diseases causing great economic loss worldwide (21, 30). These are also diseases of concern for other ruminant species and humans (8, 21). The presence of M. bovis in wild-animal reservoirs (21, 23, 32, 38, 49) has also made control of the diseases more difficult.
At present, the standard diagnostic assay for bTB is the single intradermal (SID) test (skin test) with purified protein derivative (PPD). There are many limitations to the SID test and other cell-mediated immunity (CMI)-based tests (e.g., gamma interferon [IFN-γ] and lymphocyte proliferation assays) for the diagnosis of bTB. The first limitation is that the delayed-type hypersensitivity response to the antigen has low sensitivity and specificity. It is not possible to determine if the response to PPD is attributable to exposure to M. bovis, M. avium subsp. paratuberculosis, or environmental mycobacteria, e.g., Mycobacterium avium subsp. avium. Second, there is a predominant CMI response during the early phases of infection with low bacillary loads which may be reduced or absent in animals at the advanced stages of disease with high bacterial loads. The animals become anergic to CMI-based tests (18, 35, 42, 46). Third, animals need to be handled twice over a 72-h period to obtain results of the test. Fourth, other CMI-based tests are expensive and require trained staff to perform the tests and interpret the results. Fifth, false positives may result from exposure to environmental mycobacteria and/or vaccination with BCG. Therefore, there is a need to develop sensitive and easy-to-use M. bovis-specific serological tests that can be used to distinguish between animals exposed to M. bovis, M. avium subsp. paratuberculosis, and environmental mycobacteria.
The currently available serological assays developed for herd testing have low sensitivity and suboptimal specificity (7, 35, 47), probably due to the use of complex bacterial extracts containing antigens expressed by pathogenic and environmental mycobacteria. It is possible that diagnostic assays can be improved by using antigens unique to the pathogen. Further improvement may also be achieved by the use of different diagnostic techniques. As an example, ongoing studies have shown that a latex bead agglutination assay (LBAA) has considerable potential. Increased sensitivity and specificity have been obtained with latex beads coated with ESAT6-p, a peptide containing the immunodominant portion of ESAT-6 (early secretory antigenic target) (25), a protein secreted during the early or active phase of mycobacterial infection. The antigen is not present in M. avium subsp. paratuberculosis, and the gene encoding it has been deleted in BCG (9, 52, 57, 58). Since the LBAA needs only serum, latex beads conjugated with ESAT6-p, and phosphate-buffered saline (PBS) as a diluent, it can be used to diagnose bTB in any species.
Another protein with potential for use in the LBAA and other assays is MPB70, a major secreted immunogenic protein component of tuberculin (18). It is another protein present in M. bovis culture filtrates (11, 12, 16, 34). High sensitivity and specificity have been reported in tests where the antigen was used in an enzyme-linked immunosorbent assay (EIA) and a fluorescence polarization assay (13, 14, 18, 28, 46, 55, 56, 63). The objective of the present study was to compare the sensitivity and specificity of the LBAA with the EIA using ESAT6-p or a recombinant form of MPB70 (rMPB70) containing T- and B-cell epitopes (4, 18, 28, 33, 37, 43, 48). The activity of rMPB70 was also examined for use in a commercial EIA and an immunochromatographic assay (ICGA) under patent application after development (Animal Genetics Inc., Suwon, South Korea). The results reported here show that the LBAA and ICGA with M. bovis-specific antigens offer an approach to the development of rapid diagnostic assays for use in the differential diagnosis of bTB caused by M. bovis and Johne's disease caused by M. avium subsp. paratuberculosis.
MATERIALS AND METHODS
Animals.
Three different groups of bovine and cervine sera were used in this study as follows.
M. bovis-infected cattle and deer.
Sera from naturally or experimentally M. bovis-infected cattle (n = 300 and n = 20, respectively) were used as in previous studies (25, 40). Briefly, sera were obtained from 300 cows within 10 days after a positive SID test (over 5-mm skin thickness) was observed in animals surveyed in the national herd check program. Documentation of infection with M. bovis was verified by culture of M. bovis from intestinal tissue or nasal and tracheal mucus obtained at the time of necropsy. Additionally, sera were obtained from 20 calves (5 calves in each of four groups) experimentally infected by aerosol challenge with either of two different isolates of M. bovis, one from white-tailed deer (1315) and the other from cattle (HC2005T) (40). Experimental infection of M. bovis was confirmed in 19 calves by the tuberculin skin test, isolation of M. bovis, and gross or microscopic tuberculous lesions in the lungs and tracheobronchial and mediastinal lymph nodes at the time of necropsy.
Experimental infection of four deer with M. bovis was performed by intratonsillar instillation of 2 × 108 CFU of M. bovis strain 1315. Sera from the deer were collected over a 10-month period preinfection and postinfection (p.i.) (1 week preinfection and 90, 119, 228, 252, and 309 days p.i.) (39). Infection was confirmed in all four deer by a positive tuberculin skin test, isolation of M. bovis from nasal secretions and saliva, and gross or microscopic disseminated tuberculous lesions in the lungs and tracheobronchial and mediastinal lymph nodes at the time of necropsy.
The skin test was done at 57 and 123 days postexposure to M. bovis in the aerosol-infected calves (40) and at 96 and 225 days p.i. in the intratonsillarly M. bovis-infected deer (39).
M. avium subsp. paratuberculosis-infected cattle.
Sera collected from naturally and experimentally M. avium subsp. paratuberculosis-infected cattle (n = 149 and n = 8, respectively) were used as controls in the diagnostic assays as described in previous reports (25, 61). Infection with M. avium subsp. paratuberculosis was verified by clinical signs of advanced paratuberculosis or by use of the PARACHEK, Johne's Absorbed EIA (CSL Veterinary, Parkville, Victoria, Australia) and the isolation of M. avium subsp. paratuberculosis from the intestine at necropsy or analysis of the immune response to M. avium subsp. paratuberculosis antigens in the eight experimentally infected calves (26, 61).
Since all of the M. avium subsp. paratuberculosis-positive cattle were confirmed to be free of M. bovis by bacterial culture and PCR with intestinal tissue or fecal samples, the sera were included in this study to demonstrate that there were no cross-reactive antibodies present in sera from M. avium subsp. paratuberculosis-infected cattle.
M. bovis- and M. avium subsp. paratuberculosis-free animals.
Additional control sera were obtained from healthy animals including 155 cows in the Washington State University dairy herd and the Seoul National University dairy herd that were free of M. bovis and M. avium subsp. paratuberculosis and four deer used in a previous study (39).
Preparation of ESAT6-p- and recombinant MPB70 protein (rMPB70)-conjugated latex beads.
Polystyrene microspheres with vinyl carboxylic acid (nearly soap free, 0.85 μm) from Bangs Labs Inc. (Fishers, Ind.) and the synthesized peptide (31) of ESAT-6 (KGSGSMTEQQWNFAGIEAAASAIQG) known to contain an epitope recognized by antibodies from infected animals (15, 53) were used for this study (25). An extra lysine, an extra glycine, and an extra serine were added to the N-terminal end of ESAT6-p to make an amide bond with the carboxylate groups on the beads (20).
Three kinds of rMPB70 were obtained from Animal Genetics Inc. (Suwon, South Korea) for use in this study: (i) purified rMPB70 for use as a capture antigen on the EIA plate, (ii) rPBM70 conjugated to colloidal gold for use as a detector reagent in the conjugate pad of the ICGA device, and (iii) rMPB70 used to coat the formatted test line in an ICGA device.
Different concentrations of ESAT6-p or one of the three kinds of rMPB70 were conjugated to beads using the following protocol and then tested for the capacity to agglutinate in the presence of sera from M. bovis-infected cattle. To conjugate the beads with ESAT6-p or rMPB70, the beads were washed four times, resuspended in an activation buffer (2-morpholinoethanesulfonic acid, monohydrate [pH 5.2]), and then activated by mixing with 10 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma) and 5 mM sulfo-N-hydroxysuccinimide (Sigma) (54). After mixing for 30 min on a rotating wheel, the beads were washed and resuspended in coupling buffer (borate buffer, pH 8.5) and then reacted with different concentrations of ESAT6-p dissolved in dimethyl sulfoxide or rMPB70 dissolved in water. The bead mixture was placed on the rotating wheel and mixed for 18 h at room temperature, resuspended in a blocking solution (10 mM glycine), and then mixed for an additional 30 min. In the final step, the beads were washed and then incubated for 3 h at room temperature in cold PBS (pH 7.2) containing 1% bovine serum albumin and 0.05% Tween 20. The conjugated beads were stored at 4°C until used.
To standardize the assay, a checkerboard array was used in 96-well round-bottom tissue culture plates, with different concentrations of conjugated beads (50 μl/well in PBS) mixed with different concentrations of sera (50 μl/well) from uninfected control and M. bovis-infected animals. After determining the optimal conditions for obtaining M. bovis antigen-specific agglutination, the LBAA was conducted with serial dilutions of sera from control animals and animals infected with M. avium subsp. paratuberculosis or M. bovis.
EIA with ESAT6-p.
To compare the sensitivity and specificity of the EIA with M. bovis culture and the skin test, the reference test for the diagnosis of bTB, an EIA was developed with ESAT6-p and standardized by checkerboard titration to determine the optimal concentration of ESAT6-p and serum to use in the assay (25). After developing the EIA, the assay was performed and replicated on the same day with the use of the same sera from three healthy control cows, three M. avium subsp. paratuberculosis-infected cows, and three M. bovis-infected cows in each EIA plate to determine the intra- and interassay variations of optical density (OD) values and minimize any possible variations due to the use of different plates and performance of the assay on different days. Detailed protocols for the EIA with ESAT6-p were exactly followed as those in a previous study (25).
EIA and ICGA kit with rMPB70.
The EIA kit and the ICGA kit using rMPB70 as the capture or conjugated antigen have been in the process of patent application after development at Animal Genetics Inc. Those two kits use the direct sandwich method for antibody capture by using rMPB70 with different carriers or epitopes. In the EIA kits, one kind of rMPB70 antigens has been used to coat the plate and another rMPB70 conjugated with enzyme is used instead of a secondary antibody against a specific isotype following the application of sample serum. In the ICGA platform, anti-MPB70 antibody within sample serum can be captured on an rMPB70 antigen-colloidal gold complex which is wicked through the sample pad and then trapped at a test line band containing rMPB70. If no anti-MPB70 antibody is bound to the antigen-colloidal gold complex, the complex passes through to a second control band containing a goat anti-MPB70 polyclonal antibody, where it is trapped. Two bands will appear in the ICGA exposed to sera containing anti-MPB70 antibodies. One band will appear in the ICGA exposed to sera containing no anti-MPB70 antibodies.
Any kind of rMPB70 antibody isotype from any species can be detected, as a specific secondary antibody is not used to develop the conjugate-enzyme complex (EIA) or to capture an antigen in a test line (ICGA). The protocols for the two kits, provided by the company, were followed exactly. In brief, EIA plates coated with rMPB70 were incubated with 50 μl of serum (sample and positive and negative control sera in the kit) diluted in 100 μl of diluent buffer and incubated at 37°C for 1 h, washed, and then reacted with 100 μl of diluted rMPB70-enzyme conjugate solution at 37°C for 30 min. Following washing, plates were incubated with 100 μl of diluted substrate solution at room temperature for 30 min and then 100 μl of stop solution was added to stop color development. Absorbance was read at 450 nm with 620 nm as the reference wavelength. For the ICGA kit, 20 μl of serum and 3 drops of diluent were applied to the sample pad. Results were recorded after 5 to 20 min as positive if the test and control lines were visible on the test pad and negative if only the control line was visible.
Data analysis.
Suitable cutoff values for the EIA were determined by receiver operating characteristic (ROC) analysis (3) based on the results obtained with the reference tests composed of bacterial culture and the skin test. The agreement between the positive or negative results obtained with the reference tests, the LBAA, the ICGA, and the EIA was determined by the correlation rate (CR) (51) or the kappa value from the kappa test (1). Additionally, the statistically significant association between different assays was confirmed by the chi-square test (50). The correlation between the OD value from the EIA and the intensity of agglutination or the test line band from the LBAA or ICGA was determined by the correlation coefficient from Spearman rank correlation analysis (6). All statistical analyses were carried out with commercially available (Analyze-it) software.
RESULTS
Standardization of the LBAA.
Testing of the three kinds of rMPB70 which are used as the capture antigen in the EIA (purified rMPB70), the antigen-colloidal gold complex, or the capture antigen in the test line in the ICGA revealed that the antigen-colloidal gold complex caused background agglutination due to the reaction between the beads and the antigen-colloidal gold complex. The third antigen, the capture antigen used in the ICGA, made stronger agglutination with M. bovis-positive sera compared with the purified antigen. This form of rMPB70 was selected as the optimal antigen for use in the LBAA. Testing of latex beads coated with different concentrations of ESAT6-p or rMPB70 showed that conjugation of 1 mg of beads with 45 μg of ESAT6-p or 43.56 μg of rMPB70 was the best for obtaining specific agglutination, with no agglutination evident with sera from M. bovis-negative control animals (25). Examination of plates over a 4-h period revealed that agglutination was evident with all test sera by 2 to 3 h. There was no change in the patterns of agglutination when the plates were incubated overnight at 4°C.
Comparison of sensitivity and specificity.
After determining the optimal conditions for agglutination with latex beads conjugated with ESAT6-p or rMPB70, sera from naturally and experimentally M. bovis-infected cows and deer were tested at five dilutions (1/5, 1/10, 1/20, 1/40, and 1/80) along with sera from M. bovis-negative animals. Consistent results were obtained using a dilution of 1/10 to 1/20 for LBAA with ESAT6-p and rMPB70. The intensity of agglutination was calculated and analyzed in 10 fields of 1 mm2 randomly selected in wells of the LBAA (41) with the image analyzer (Olympus, Melville, N.Y.) and Optimas 6.5 programs. Agglutination was stronger with some sera than others, and agglutination levels of 0 to 25%, 26 to 50%, 51 to 75%, and 76 to 100% were categorized as 1+, 2+, 3+, and 4+ (positive), respectively (25). Although background agglutination was noted at higher concentrations of serum with some infected animals, little agglutination was evident with the test sera from control animals. In the same way as in the LBAA, the intensity of the test line band in the ICGA was also analyzed and categorized as 1+, 2+, 3+, or 4+ (positive).
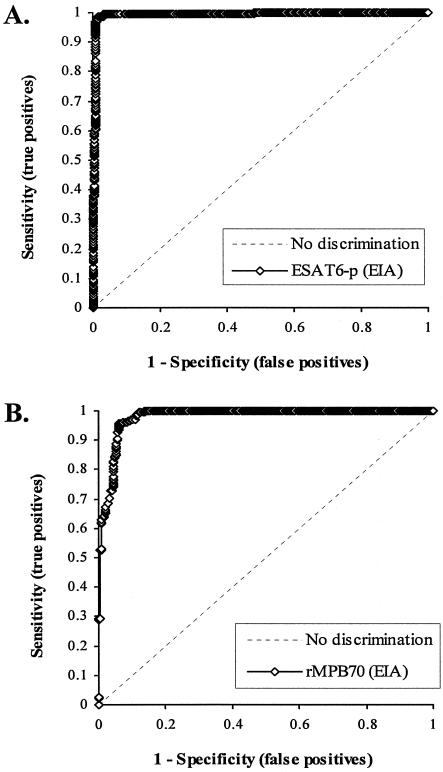
To compare sensitivity and specificity between the different assay formats and between assays using ESAT6-p and rMPB70, the same sera were first tested in the EIA (both antigens) and ICGA (rMPB70 only) formats. When the ODs of all the sera from cattle and deer were applied to ROC analysis (Fig. 1-A), the ROC curve from the EIA with ESAT6-p had an area under the concentration-time curve (AUC) of 0.995 (95% confidence interval [CI], 0.990 to 1.000, P < 0.01) and a standard error (SE) of the AUC of 0.0027. For the EIA with rMPB70, the AUC and SE were 0.980 (95% CI, 0.970 to 0.989, P < 0.01) and 0.0047, respectively (Fig. 1B). Among different cutoff values from the ROC analysis, 0.5 and 0.045 were selected because sensitivities of 98.6 and 96.8% and specificities of 98.5 and 90.1% were optimal for the EIA with ESAT6-p and rMPB70, respectively. All the cervine sera from the four experimentally M. bovis-infected deer and 293 out of 324 bovine sera from naturally or experimentally M. bovis-infected cattle showed ODs greater than the selected cutoff values in the EIA with rMPB70 (Table 1). Out of M. bovis-positive sera from cattle and deer, only four sera from aerosol M. bovis-challenged calves were negative in the EIA with ESAT6-p.
FIG. 1.
ROC curves for EIA with ESAT6-p (A) or rMPB70 (B). Sensitivity and specificity, respectively, were calculated as the number of serum samples showing positive results by the reference test and the EIA at each cutoff value divided by the number of reference test positive results and as the number of serum samples showing negative results by the reference test and the EIA at each cutoff value divided by the number of reference test negative results, based on the results of M. bovis culture or a skin test as the reference test and a comparison of those two tests (reference test versus EIA).
TABLE 1.
Overall serological responses of sera from naturally or experimentally M. bovis- or M. avium subsp. paratuberculosis-infected or -free cattle or deer in an EIA, an ICGA, or an LBAA utilizing rMPB70 or ESAT6-p as the coating or conjugating antigena
| Antigen and assay | No. of positive sera/no. testedd
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
M. bovis-infected animalsa
|
M. bovis-free animalsb
|
|||||||
| Cattle
|
Deer | Total | M. avium subsp. paratuberculosis free | M. avium subsp. paratuberculosis infected | Total | |||
| Naturally infectedc | Experimentally inoculated | Total | ||||||
| rMBP70 | ||||||||
| EIA | 272/300 (90.7) | 17/20 (85.0) | 289/320 (90.3) | 4/4 (100) | 293/324 (96.8) | 5/155 (96.8) | 1/157 (99.4) | 6/312 (90.1) |
| ICGA | 248/300 (82.7) | 18/20 (90.0) | 266/320 (83.1) | 3/4 (75.0) | 269/324 (83.0) | 2/155 (98.7) | 0/157 (100) | 2/312 (99.4) |
| LBAA | 259/300 (86.3) | 18/20 (90.0) | 277/320 (86.6) | 4/4 (100) | 281/324 (86.7) | 5/155 (96.8) | 2/157 (98.7) | 7/312 (97.8) |
| ESAT6 | ||||||||
| EIA | 300/300 (100) | 16/20 (80.0) | 316/320 (98.8) | 4/4 (100) | 320/324 (98.6) | 4/155 (97.4) | 4/155 (97.4) | 4/312 (98.5) |
| LBAA | 283/300 (94.3) | 20/20 (100) | 303/320 (94.7) | 4/4 (100) | 307/324 (94.8) | 15/155 (90.3) | 15/155 (90.3) | 23/312 (92.6) |
The group of M. bovis-infected animals (n = 324) included cattle (n = 320) (both naturally infected cows [n = 300] and experimentally inoculated calves [n = 20] and experimentally inoculated deer (n = 4). Values in parentheses are sensitivities (given as percentages).
The group of M. bovis-free animals (n = 312) included both M. avium subsp. paratuberculosis-free animals (n = 155) and M. avium subsp. paratuberculosis-infected cattle (n = 157). Values in parentheses are specificities (given as percentages).
Natural M. bovis infection was checked by the SID test (over 5-mm skin thickness after the test) and culture of M. bovis from intestinal tissue or nasal and tracheal mucus at the time of necropsy.
Detailed information on how natural M. bovis or M. avium subsp. paratuberculosis infection was confirmed, how M. bovis or M. avium subsp. paratuberculosis was inoculated experimentally, and how a positive response in the EIA, LBAA, or ICGA was defined is given in Materials and Methods. Although one M. bovis-inoculated calf with NVL and three deer at the NVL stage (90 days p.i.) were negative by M. bovis culture, their sera showed a positive response with ESAT6-p by the EIA and LBAA, and serum from one NVL calf also reacted positively with rMPB70 by the LBAA. The specificity of the EIA with ESAT6-p or rMPB70 was also affected because the specificity of the EIA was calculated by comparing the result of bacterial culture or a skin test with that of the EIA by ROC analysis with the Analyse-it program. The EIA and the ICGA kit using rMPB70 were obtained from Animal Genetics Inc. (Suwon, South Korea), and the other assays (the EIA with ESAT6-p and the LBAA) were developed in this study.
Serological assays with rMPB70 and ESAT6-p for bTB showed similar sensitivities (96.8, 83.0, and 86.7% for the EIA, ICGA, and LBAA using rMPB70, respectively, and 98.6 and 94.8% for the EIA and LBAA with ESAT6-p, respectively; Table 1).
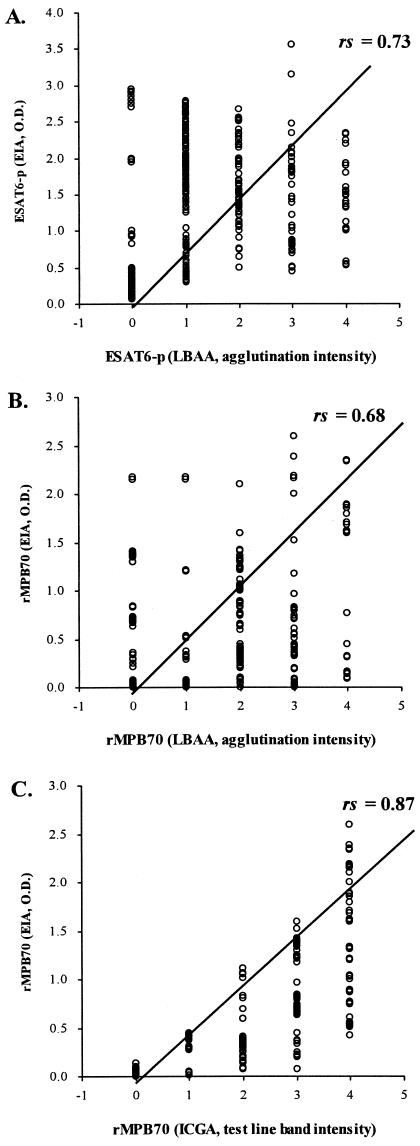
The CRs (51), the kappa (Κ) values from the kappa test (1), and the χ2 values from the chi-square analysis (50) were calculated to evaluate the agreement between the positive and negative results obtained with the reference test, the LBAA, the ICGA, and the EIA (Table 2). After Spearman rank correlation analysis (6), the correlation coefficient (r) between the OD value from the EIA and the intensity of agglutination in the LBAA was 0.73 (95% CI, 0.70 to 0.77, P < 0.0001) for ESAT6-p and 0.68 (95% CI, 0.64 to 0.72, P < 0.0001) for rMPB70 (Fig. 2A and B). The intensity of the test line band in the ICGA was also highly correlated with the OD value from the EIA utilizing rMPB70 with an r of 0.87 (95% CI, 0.85 to 0.88, P < 0.0001) (Fig. 2C).
TABLE 2.
Comparison of the diagnostic tests used in this study
| Test or antigen and assay | CR or K value (χ2 value)a
|
|||||
|---|---|---|---|---|---|---|
| Culture or skin test | ESAT6-p
|
rMPB70
|
||||
| EIA | LBAA | EIA | LBAA | ICGA | ||
| Culture or skin test | 0.97 (628.57) | 0.88 (510.73) | 0.87 (510.62) | 0.85 (480.72) | 0.83 (469.80) | |
| ESAT6-p | 0.88 (514.18) | |||||
| EIA | 98.7 | 0.86 (493.47) | 0.84 (470.87) | 0.81 (446.73) | ||
| LBAA | 93.9 | 94.0 | 0.77 (396.96) | 0.75 (382.91) | 0.74 (373.49) | |
| rMPB70 | ||||||
| EIA | 93.7 | 92.9 | 83.5 | 0.74 (358.01) | 0.91 (557.06) | |
| LBAA | 92.4 | 91.9 | 87.7 | 87.0 | 0.70 (321.33) | |
| ICGA | 91.6 | 90.6 | 87.0 | 95.8 | 85.2 | |
CRs (percent) are in boldface type. The K values and χ2 values were obtained by chi-square analysis performed to evaluate the agreement between a positive or negative result of a bacterial culture or skin test, an EIA, an LBAA, and an ICGA with ESAT6-p or rMPB70. According to the chi-square analysis, all of the assays used in this study are statistically significantly associated with each other (P < 0.0001).
FIG. 2.
Spearman rank correlation analysis between OD values from the EIA and the intensity of agglutination from the LBAA utilizing ESAT-6 (A) or rMPB70 (B) and between OD values and the intensity of the test line band from the ICGA with rMPB70 (C). The EIA and LBAA turned out to be positively correlated (P < 0.0001), with r = 0.73 (95% CI, 0.70 to 0.77) or 0.68 (95% CI, 0.64 to 0.72) when using ESAT-6 or rMPB70 as the detecting antigen, respectively. A statistically significant correlation between the ICGA and EIA utilizing rMPB70 was also found, with r = 0.87 (95% CI, 0.85 to 0.88, P < 0.0001).
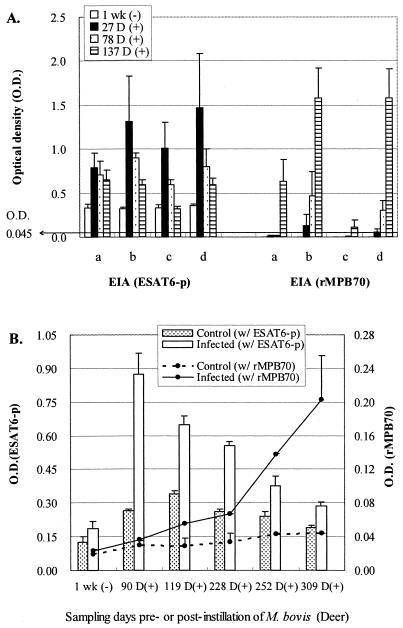
The assays with rMPB70 detected M. bovis-positive sera that were negative in the ESAT6-p assays. With the sera collected four or five times, monthly or bimonthly, from experimentally M. bovis-infected calves and deer, assays utilizing ESAT6-p could detect antibody activity against M. bovis in samples collected at earlier time points compared with assays with rMPB70 (Tables 3 and 4; Fig. 3). None of the sera from the preinfection stage of cattle and deer experimentally infected with M. bovis showed a positive reaction in the EIA, LBAA, or ICGA.
TABLE 3.
Summary of serological responses of sera sampled monthly or bimonthly after inoculation from experimentally M. bovis-infected calves in an EIA, ICGA, or LBAA utilizing rMPB70 or ESAT6-p as the capture or conjugating antigen
| Sample datea | ESAT6-p
|
rMPB70
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EIA
|
LBAA
|
EIA
|
LBAA
|
ICGA
|
|||||||||||||||||||||
| Value for groupb:
|
Totalc | Value for groupb:
|
Total | Value for groupb:
|
Total | Value for groupb:
|
Total | Value for groupb:
|
Total | ||||||||||||||||
| a | b | c | d | a | b | c | d | a | b | c | d | a | b | c | d | a | b | c | d | ||||||
| 2 wks (−) | 0 | 0 | 0 | 0 | 0/20 (0) | 0 | 0 | 0 | 0 | 0/20 (0) | 0 | 0 | 0 | 0 | 0/20 (0) | 0 | 0 | 0 | 0 | 0/20 (0) | 0 | 0 | 0 | 0 | 0/20 (0) |
| 27 days (+) | 3 | 4 | 4 | 3 | 14/20 (70) | 2 | 4 | 5 | 5 | 16/20 (80) | 0 | 1 | 0 | 1 | 2/20 (10) | 2 | 5 | 1 | 3 | 11/20 (55) | 0 | 3 | 0 | 3 | 6/20 (30) |
| 78 days (+) | 2 | 0 | 0 | 2 | 4/20 (20) | 4 | 5 | 4 | 5 | 18/20 (90) | 0 | 4 | 0 | 4 | 8/20 (40) | 2 | 5 | 2 | 5 | 14/20 (70) | 1 | 5 | 2 | 5 | 13/20 (65) |
| 137 days (+) | 3 | 0 | 0 | 3 | 6/20 (30) | 5 | 0 | 0 | 3 | 8/20 (40) | 4 | 5 | 2 | 5 | 16/20 (80) | 3 | 5 | 4 | 5 | 17/20 (85) | 5 | 5 | 3 | 5 | 18/20 (90) |
(−), preinfection; (+), postinfection.
Experimental M. bovis infection of 20 calves (5 calves in each of four groups) was carried out by aerosol challenge of 103 (group a) or 105 (group b) CFU of M. bovis from white-tailed deer (1315) or 103 (group c) or 105 (group d) CFU of M. bovis from cattle (HC2005T) (40). Values are numbers of positive sera.
Values are numbers of positive sera/numbers of sera tested, with percent sensitivity in parentheses. ESAT6-p shows a positive reaction with sera collected in the earlier months, but rMPB70 detects positive sera collected in the later months. CMI to M. bovis tuberculin was checked by a skin test with aerosol-infected calves at 57 and 123 days postchallenge with M. bovis (40). As in other studies (27, 28), those skin tests gave a positive effect on the titer of antibody to rMPB70 but not ESAT6-p in blood samples taken 2 and 3 weeks following the skin test.
TABLE 4.
Summary of serological responses of sera sampled monthly or bimonthly after inoculation from experimentally M. bovis-infected deer in an EIA, ICGA, or LBAA utilizing rMPB70 or ESAT6-p as the capture or conjugating antigena
| Sample dateb | EIA with ESAT6-p | LBAA with ESAT6-p | EIA with rMPB70 | LBAA with rMPB70 | ICGA with rMPB70 |
|---|---|---|---|---|---|
| 2 wks (−) | 0/4 (0) | 0/4 (0) | 0/4 (0) | 0/4 (0) | 0/4 (0) |
| 90 days (+) | 4/4 (100) | 4/4 (100) | 0/4 (0) | 0/4 (0) | 0/4 (0) |
| 119 days (+) | 4/4 (100) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) |
| 228 days (+) | 2/4 (50) | 2/4 (50) | 4/4 (100) | 1/4 (25) | 0/4 (0) |
| 252 days (+) | 0/2 (0) | 0/2 (0) | 2/2 (100) | 0/2 (0) | 0/2 (0) |
| 309 days (+) | 0/4 (0) | 0/4 (0) | 4/4 (100) | 3/4 (75) | 2/4 (50) |
Values are numbers of positive sera/numbers of sera/tested, with percent sensitivity in parentheses. ESAT6-p shows a positive reaction with sera collected in the earlier month, but rMPB70 detects positive sera collected in the later month. CMI to M. bovis tuberculin was checked by a skin test with intratonsillarly M. bovis-infected deer at 96 and 225 days postinfection (39). As in other studies (27, 28), those skin tests gave a positive effect on the titer of antibody to rMPB70 but not ESAT6-p in blood samples taken 2 and 3 weeks following the skin test.
(−), preinfection; (+), postinfection.
FIG. 3.
ODs from the EIA with ESAT6-p or rMPB70 and sera from experimentally M. bovis-infected calves (A) or deer and control deer (B). Detailed information on how M. bovis was inoculated experimentally into calves or deer and how blood was collected from them is given in Materials and Methods. ODs from EIAs using different capture or detecting antigens and sera from control or M. bovis-infected deer are shown on the y axes (EIA using ESAT-6 on the left y axis and EIA with rMPB70 on the right y axis [B]). D, days; wk, week.
DISCUSSION
In the early or active phase of infection, the metabolically active mycobacteria secrete proteins such as ESAT-6 and CFP10 antigens, which were originally identified in culture filtrates of M. tuberculosis containing highly immunogenic low-molecule-mass proteins. The genes encoding these proteins are located in the same operon (9, 52). The antigens elicit a strong antibody response and induce secretion of IFN-γ. The response to these antigens has been found in recent converters to PPD positivity and in tuberculosis patients but not in BCG-vaccinated or unvaccinated individuals (57). ESAT-6 (6 kDa) is virtually specific for the M. tuberculosis complex; the gene is present in all isolates of M. tuberculosis and virulent M. bovis, but it is absent from all strains of BCG (52). Therefore, ESAT-6 and CFP10 can be potential candidates for use in early detection as substitutes or as improved skin test antigens (58). In particular, ESAT-6 is an important T-cell antigen recognized by protective T cells in early stages of M. tuberculosis infection in an animal model (52). A monoclonal antibody, HYB76-8, has been known to be specific to two peptides (p1-20 and p12-35) in the N-terminal region of the ESAT-6 protein in which p1-20 peptides (MTEQQWNFAGIEAAASAIQG) most strongly react with the monoclonal antibody (15, 17). As a result, high specificity could be achieved by using synthetic ESAT6-p containing sequences from native proteins known to contain an epitope recognized by antibodies in sera from infected animals.
MPB70 is one of the immunodominant antigens of M. bovis (11, 12). It is an extremely stable and active component of bovine tuberculin (18). Skin testing with tuberculin boosts the immunoglobulin G1 antibody responses to MPB70 (28), particularly antibody recognizing the epitope within residues 51 to 70 of MPB70 in skin test-positive cattle. This memory response has been found in M. bovis-infected cattle with macroscopic and tuberculous lesions (27). It is able to elicit a strong delayed-type hypersensitivity response and to stimulate T-lymphocyte proliferation and antibody production in M. bovis-infected animals (10, 11, 16, 18, 19, 43). Effective DNA vaccination of cattle with mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test (60). Moreover, a plasmid expressing MPB70 has been shown to be a better therapeutic vaccine in mice than a plasmid expressing hsp65, particularly against the growth of bacteria in the lung (29).
For the development of simple and easy-to-use agglutination assays, latex bead technology has been used successfully (24, 36, 59, 64, 65). Similarly, recombinant or synthetic peptides derived from species-specific M. bovis proteins containing epitopes recognized by antibodies from infected animals can be used singly or multiplexed on a lysine polymer backbone using currently available technology to detect antibodies specific for M. bovis (22).
Comparisons between the reference test and the assays used or developed in this study were made by calculating the CR, Κ value, and r after Spearman rank correlation analysis and confirmed by chi-square analysis (Fig. 2; Table 2). Comparison of the reference test and assays with ESAT6-p gave Κ values of 0.97 and 0.88 and CRs of 98.7 and 93.9% for the EIA and LBAA, respectively (P < 0.0001) (Table 2). The high values of Κ and CR indicate that EIA and LBAA with ESAT6-p can be used as reliable methods for detection of animals infected with M. bovis. Results with the prototype of the EIA and ICGA utilizing rMPB70, developed at Animal Genetics Inc., yielded results very similar to those obtained with the reference test (Κ values of 0.87 and 0.83 and CRs of 93.7 and 91.6%, respectively). The LBAA with rMPB70 showed a K value (0.85) and CR (92.4%) comparable to those of the two kinds of prototype assays. This means that the LBAA utilizing rMPB70 has a similar sensitivity, and it can be used as well as the prototype assays from Animal Genetics Inc. for the diagnosis of bTB.
Serial samples of sera collected at pre- and postinfection were applied to the EIA, ICGA, and LBAA to determine how early an antibody response to ESAT6-p and rMPB70 can be detectable and how its dynamic changes occur at different stages of M. bovis infection (Tables 3 and 4; Fig. 3). Although all of the preinfection sera were negative in the EIA and LBAA, 80%, 90%, and 90% of the bovine sera collected at 27, 78, and 137 days following an aerosol challenge with M. bovis showed positive serological responses to ESAT6-p and rMPB70 in the EIA, LBAA, and ICGA, respectively (Tables 3 and 4). A positive antibody response to ESAT6 was first detected in samples of blood from experimentally M. bovis-infected calves and deer at 27 and 90 days p.i., respectively. Antibody activity was consistently detectable in M. bovis-infected calves until 137 days p.i. in all groups except group c, where antibody activity became less detectable by 78 days. Animals in this group were infected with 103 CFU of M. bovis (HC2005T, cattle strain). Antibody activity was detectable in infected deer until 228 days p.i. In contrast to ESAT6-p, antibody activity to rMPB70 appeared at 27 days p.i. in calves which were infected with 105 CFU of M. bovis (groups b and d) and at 137 days p.i. in calves infected with 103 CFU of M. bovis (groups a and c). Antibody was detected at 119 days p.i. in deer. The titer of the antibody increased after its first appearance, and it was still present at a high titer until the last blood sampling day (137 and 309 days p.i. for calves and deer, respectively). Infection with a low or high dose of M. bovis appeared to affect the timing of when antibody responses to ESAT6-p and rMPB70 were detectable.
In a diseased herd, infected animals must be identified and slaughtered. This becomes difficult as skin tests are not very sensitive and only a third of infected animals show lesions. Small granulomas may be seen when the lymph nodes are cut into thin sections. Animals with nonvisible lesions (NVL) are those that are positive by skin test with no visible lesions and M. bovis culture negative. On the other hand, the heavily infected animals may not react in the skin test, a situation referred to as “anergy.” However, NVL cattle and anergic cattle in the advanced stage of bTB exhibit a striking immunoglobulin G1 memory response to rMPB70 (27, 28), as demonstrated in the memory index ratio of antibody titer detected before and after skin testing. It has also been shown in this study with one of the NVL calves experimentally infected by aerosol exposure (103 CFU of M. bovis) that showed no gross or microscopic lesions, no response to the tuberculin skin test, and no outgrowth of bacteria from feces, lymph nodes, and other tissues (40). Serum from this calf was positive for ESAT6-p in the EIA and LBAA developed in this study by day 27 p.i., and it became positive for both ESAT6-p and rMPB70 by the LBAA at 78 and 137 days p.i. Sera collected from this NVL calf even at 137 days p.i. and from three deer out of a total of four deer at 90 days p.i. showed ODs higher than or the same as the determined cutoff value (0.5 for ESAT6-p and 0.045 for rMPB70) in the EIA.
Skin tests at 57 and 123 days p.i. in infected calves (40) and at 96 and 225 days p.i. in intratonsillarly M. bovis-infected deer (39) had a boosting effect on the titer of antibody to rMPB70, but not ESAT6-p, in blood samples taken 2 and 3 weeks following a skin test, as expected (27, 28). In summary, it can be concluded that the anti-ESAT6-p antibody titer might decrease during disease progression while the titer of antibody to MPB70 increases (27). Consequently, the serological assays (EIA, LBAA, and ICGA) developed and used in this study should be useful in the detection of NVL cattle infected with M. bovis early in the course of disease and later, with the use of the memory index of antibody response to rMPB70 following skin testing.
In the present study, we have demonstrated that the LBAA and ICGA using ESAT6-p or rMPB70 offer the potential for developing relatively rapid assays for detecting animals infected with M. bovis. Additional advantages that LBAA and ICGA offer are that the same assays can be used in multiple species since no additional reagents are necessary to develop the test and the results can be interpreted within 2 or 3 h with the LBAA and 20 min with the ICGA. The three kinds of rMPB70 antigens used as the capture antigens for the EIA, the conjugated antigen with colloidal gold for the ICGA, and the capture antigen used at the test line of the ICGA are all different in terms of different carrier or epitopes. Moreover, the secondary reagent, used after the reaction between serum antibody and rMPB70 used to coat the EIA plate or rMPB70 conjugated with colloidal gold in the sample pad of the ICGA kit, is an rMPB70-conjugated enzyme or another rMPB70 antigen in each assay, respectively. This suggests that sera from any species can be tested with the EIA and ICGA kits from Animal Genetics Inc. These two kits can detect all of the isotypes of anti-MPB70 antibody by enzyme reaction or antigen-colloidal gold complex.
Additionally, the EIA and ICGA or LBAA detecting anti-MPB70 antibodies have increased the sensitivity for diagnosis of bTB to 99.7 and 100% when combined with each assay to detect antibody to ESAT6-p. It will be worthwhile to determine if the use of a cocktail of antigens made of ESAT6-p and rMPB70 for detecting bTB infection may be effective in identifying animals regardless of the stage of disease. Separate assays could be subsequently used to differentiate infected animals at the early and advanced stages of disease.
The accuracy of these combined assays or each test used in this study, which is demonstrated as sensitivity and specificity (Table 1), is higher than or comparable to that in other studies. An EIA with nine purified recombinant proteins of M. tuberculosis complex mycobacteria, including ESAT-6 and MPB70, could detect antibody-positive reactors composed of 16 out of 28 IFN-γ-positive cattle from field bTB outbreaks (2). Additionally, the Mantoux test (tuberculin skin test) and the tuberculin IFN-γ assay (Quanti-FERON-TB) were reported to have sensitivities of 87 and 83 to 90% and specificities of 80 and 98%, respectively (45). In Whipple's study, the caudal fold tuberculin test and IFN-γ assay showed sensitivities ranging from 80.4 to 84.4% and 55.4 to 97.1%, respectively, depending on the standard of comparison and the method of interpretation (62). According to two other different studies (5, 44), IFN-γ blood tests using PPD and ESAT-6 antigen had sensitivities ranging from 89 to 98% and 76 to 88%, depending on the cutoff values used. The specificity turned out to be 85 to 94% when using PPD for the test antigen and 94 to 100% for IFN-γ blood tests with ESAT-6 as the antigen.
In conclusion, serological assays, EIA, ICGA, and LBAA utilizing ESAT6-p for early bTB detection and rMPB70 for detection of the advanced stages of bTB, can be used with the skin test to determine the status of disease and reduce the frequency of misdiagnosis of animals free of bTB.
Acknowledgments
This study was funded in part by grants from the USDA-NRICGP (2002-35204-11688 and 2003-05165) and USDA-APHIS-VS (03-9100-0788-GR and 03-9100-07-GR), intramural support from USDA Animal Health (WNV-00150), and a Korea Research Foundation grant (KRF-005-E00076). Further support was provided by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University. This study was also supported by the Brain-Korea 21 Project in Agricultural Biotechnology and the Washington State University Monoclonal Antibody Center.
REFERENCES
- 1.Altman, D. G. 1991. Practical statistics for medical research, p. 403-409. Chapman & Hall, Ltd., London, United Kingdom.
- 2.Amadori, M., K. P. Lyashchenko, M. L. Gennaro, J. M. Pollock, and I. Zerbini. 2002. Use of recombinant proteins in antibody tests for bovine tuberculosis. Vet. Microbiol. 85:379-389. [DOI] [PubMed] [Google Scholar]
- 3.Beck, J. R., and E. K. Schultz. 1986. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch. Pathol. Lab. Med. 110:13-20. [PubMed] [Google Scholar]
- 4.Billman-Jacobe, H., A. J. Radford, J. S. Rothel, and P. R. Wood. 1990. Mapping of the T and B cell epitopes of the Mycobacterium bovis protein MPB70. Immunol. Cell Biol. 68:359-365. [DOI] [PubMed] [Google Scholar]
- 5.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2000. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 6.Conover, W. J. 1980. Practical non-parametric statistics, 2nd edition, p. 252-256. John Wiley & Sons, Inc., New York, N.Y.
- 7.Costello, E., J. W. Egan, Quigley F. C., and P. F. O'Reilly. 1997. Performance of the single intradermal comparative tuberculin test in identifying cattle with tuberculous lesions in Irish herds. Vet. Rec. 141:222-224. [DOI] [PubMed] [Google Scholar]
- 8.Daborn, C. J., and J. M. Grange. 1993. HIV/AIDS and its implications for the control of animal tuberculosis. Br. Vet. J. 149:405-417. [DOI] [PubMed] [Google Scholar]
- 9.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fifis, T., C. Costopoulos, L. A. Corner, and P. R. Wood. 1992. Serological reactivity to Mycobacterium bovis protein antigens in cattle. Vet. Microbiol. 30:343-354. [DOI] [PubMed] [Google Scholar]
- 11.Fifis, T., C. Costopoulos, A. J. Radford, A. Bacic, and P. R. Wood. 1991. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect. Immun. 59:800-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fifis, T., P. Plackett, L. A. Corner, and P. R. Wood. 1989. Purification of a major Mycobacterium bovis antigen for the diagnosis of bovine tuberculosis. Scand. J. Immunol. 29:91-101. [DOI] [PubMed] [Google Scholar]
- 13.Gaborick, C. M., M. D. Salman, R. P. Ellis, and J. Triantis. 1996. Evaluation of a five-antigen ELISA for diagnosis of tuberculosis in cattle and Cervidae. J. Am. Vet. Med. Assoc. 209:962-966. [PubMed] [Google Scholar]
- 14.Griffin, J. F., S. Nagai, and G. S. Buchan. 1991. Tuberculosis in domesticated red deer: comparison of purified protein derivative and the specific protein MPB70 for in vitro diagnosis. Res. Vet. Sci. 50:279-285. [DOI] [PubMed] [Google Scholar]
- 15.Harboe, M., A. S. Malin, H. S. Dockrell, H. G. Wiker, G. Ulvund, A. Holm, M. C. Jorgensen, and P. Andersen. 1998. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect. Immun. 66:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harboe, M., S. Nagai, M. E. Patarroyo, M. L. Torres, C. Ramirez, and N. Cruz. 1986. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect. Immun. 52:293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harboe, M., H. G. Wiker, J. R. Duncan, M. M. Garcia, T. W. Dukes, B. W. Brooks, C. Turcotte, and S. Nagai. 1990. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 28:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslov, K., A. B. Andersen, and M. W. Bentzon. 1987. Biological activity in sensitized guinea pigs of MPB 70, a protein specific for some strains of Mycobacterium bovis BCG. Scand. J. Immunol. 26:445-454. [DOI] [PubMed] [Google Scholar]
- 20.Hermanson, G. T., A. K. Mallia, and P. K. Smith. 1992. Immobilized affinity ligand techniques. Academic Press, Inc., San Diego, Calif.
- 21.Hines, M. E., J. M. Kreeger, and A. J. Herron. 1995. Mycobacterial infections of animals: pathology and pathogenesis. Lab. Anim. Sci. 45:334-351. [PubMed] [Google Scholar]
- 22.Huang, W., B. Nardelli, and J. P. Tam. 1994. Lipophilic multiple antigen peptide system for peptide immunogen and synthetic vaccine. Mol. Immunol. 31:1191-1199. [DOI] [PubMed] [Google Scholar]
- 23.Hunter, D. L. 1996. Tuberculosis in free-ranging, semi free-ranging and captive cervids. Rev. Sci. Tech. 15:171-181. [DOI] [PubMed] [Google Scholar]
- 24.Inzana, T. J., J. Todd, C. Koch, and J. Nicolet. 1992. Serotype specificity of immunological assays for the capsular polymer of Actinobacillus pleuropneumoniae serotypes 1 and 9. Vet. Microbiol. 31:351-362. [DOI] [PubMed] [Google Scholar]
- 25.Koo, H. C., Y. H. Park, J. S. Ahn, W. R. Waters, M. J. Hamilton, G. M. Barrington, A. A. Mosaad, M. V. Palmer, S. J. Shin, and W. C. Davis. 2004. New latex bead agglutination assay for differential diagnosis of cattle infected with Mycobacterium bovis and Mycobacterium avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo, H. C., Y. H. Park, M. J. Hamilton, G. M. Barrington, J. D. Christopher, J. B. Kim, and W. C. Davis. 2004. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect. Immun. 72:6870-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lightbody, K. A., J. McNair, S. D. Neill, and J. M. Pollock. 2000. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70 in immunised and in tuberculin skin test-reactor cattle. Vet. Microbiol. 75:177-188. [DOI] [PubMed] [Google Scholar]
- 28.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 29.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 30.Martin, S. W., R. A. Dietrich, P. Genho, W. P. Heuschele, R. L. Jones, M. Koller, J. D. Lee, H. C. Lopez, H. W. Moon, R. A. Robinson, P. L. Smith, and G. W. Williams. 1994. Livestock disease eradication: evaluation of the cooperative state-federal bovine tuberculosis eradication program, p. 1-97. National Academy of Sciences, Washington, D.C.
- 31.Merrifield, R. B. 1969. Solid-phase peptide synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 32:221-296. [DOI] [PubMed] [Google Scholar]
- 32.Mirsky, M. L., D. Morton, J. W. Piehl, and H. Gelberg. 1992. Mycobacterium bovis infection in a captive herd of Sika deer. J. Am. Vet. Med. Assoc. 200:1540-1542. [PubMed] [Google Scholar]
- 33.Mustafa, A. S., H. A. Amoudy, H. G. Wiker, A. T. Abal, P. Ravn, F. Oftung, and P. Andersen. 1998. Comparison of antigen-specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand. J. Immunol. 48:535-543. [DOI] [PubMed] [Google Scholar]
- 34.Nagai, S., J. Matsumoto, and T. Nagasuga. 1981. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect. Immun. 31:1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neill, S. D., J. Cassidy, J. Hanna, D. P. Mackie, J. M. Pollock, A. Clements, E. Walton, and D. G. Bryson. 1994. Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon-gamma. Vet. Rec. 135:134-135. [DOI] [PubMed] [Google Scholar]
- 36.Nisengard, R. J., L. Mikulski, D. McDuffie, and P. Bronson. 1992. Development of a rapid latex agglutination test for periodontal pathogens. J. Periodontol. 63:611-617. [DOI] [PubMed] [Google Scholar]
- 37.Oettinger, T., M. Jorgensen, A. Ladefoged, K. Haslov, and P. Andersen. 1999. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber. Lung Dis. 79:243-250. [DOI] [PubMed] [Google Scholar]
- 38.Palmer, M. V., D. L. Whipple, J. B. Payeur, D. P. Alt, K. J. Esch, F. Bruning, and J. B. Kaneene. 2000. Naturally occurring tuberculosis in white-tailed deer. J. Am. Vet. Med. Assoc. 216:1921-1924. [DOI] [PubMed] [Google Scholar]
- 39.Palmer, M. V., D. L. Whipple, and W. R. Waters. 2001. Experimental deer-to-deer transmission of Mycobacterium bovis. Am. J. Vet. Res. 62:692-696. [DOI] [PubMed] [Google Scholar]
- 40.Palmer, M. V., W. R. Waters, and D. L. Whipple. 2002. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis 82:275-282. [DOI] [PubMed] [Google Scholar]
- 41.Perez, J., J. M. de las Mulas, F. C. De Lara, P. N. Gutierrez-Palomino, C. Becerra-Martel, and A. Martinez-Moreno. 1998. Immunohistochemical study of the local immune response to Fasciola hepatica in primarily and secondarily infected goats. Vet. Immunol. Immunopathol. 64:337-348. [DOI] [PubMed] [Google Scholar]
- 42.Plackett, P., J. Ripper, L. A. Corner, K. Small, K. de Witte, L. Melville, S. Hides, and P. R. Wood. 1989. An ELISA for the detection of anergic tuberculous cattle. Aust. Vet. J. 66:15-19. [DOI] [PubMed] [Google Scholar]
- 43.Pollock, J. M., A. J. Douglas, D. P. Mackie, and S. D. Neill. 1994. Identification of bovine T-cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology 82:9-15. [PMC free article] [PubMed] [Google Scholar]
- 44.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 45.Pottumarthy, S., A. J. Morris, A. C. Harrison, and V. C. Wells. 1999. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J. Clin. Microbiol. 37:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritacco, V., B. Lopez, I. N. De Kantor, L. Barrera, F. Errico, and A. Nader. 1991. Reciprocal cellular and humoral immune responses in bovine tuberculosis. Res. Vet. Sci. 50:365-367. [DOI] [PubMed] [Google Scholar]
- 47.Ritacco, V., B. Lopez, L. Barrera, A. Nader, E. Fliess, and K. De. 1990. Further evaluation of an indirect enzyme-linked immunosorbent assay for the diagnosis of bovine tuberculosis. Zentbl. Veterinaermed. B 37:19-27. [DOI] [PubMed] [Google Scholar]
- 48.Roche, P. W., J. A. Triccas, D. T. Avery, T. Fifis, H. Billman-Jacobe, and W. J. Britton. 1994. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J. Infect. Dis. 170:1326-1330. [DOI] [PubMed] [Google Scholar]
- 49.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, F. Bruning, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 50.Siegel, S., and N. J. Castellan, Jr. 1988. Non-parametric statistics for the behavioral sciences, 2nd edition. McGraw-Hill, New York, N.Y.
- 51.Silva, E. 2001. Evaluation of an enzyme-linked immunosorbent assay in the diagnosis of bovine tuberculosis. J. Vet. Microbiol. 78:111-117. [DOI] [PubMed] [Google Scholar]
- 52.Skjot, R. L. V., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staros, J. V., R. W. Wright, and D. M. Swingle. 1986. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220-222. [DOI] [PubMed] [Google Scholar]
- 55.Sugden, E. A., K. Stilwell, E. B. Rohonczy, and P. Martineau. 1997. Competitive and indirect enzyme-linked immunosorbent assays for Mycobacterium bovis infections based on MPB70 and lipoarabinomannan antigens. Can. J. Vet. Res. 61:8-14. [PMC free article] [PubMed] [Google Scholar]
- 56.Surujballi, O. P., A. Romanowska, E. A. Sugden, C. Turcotte, and M. E. Jolley. 2002. A fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in cattle sera. Vet. Microbiol. 87:149-157. [DOI] [PubMed] [Google Scholar]
- 57.Ulrichs, T., P. Anding, S. Porcell, S. H. E. Kaufmann, and M. E. Munk. 2000. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 68:6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Pinxteren, L. A. H., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verloo, D., W. Holland, L. N. My, N. G. Thanh, P. T. Tam, V. Goddeeris, J. Vercruysse, and P. Buscher. 2000. Comparison of serological tests for Trypanosoma evansi natural infections in water buffaloes from North Vietnam. Vet. Parasitol. 20:87-96. [DOI] [PubMed] [Google Scholar]
- 60.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, M. A. Chambers, D. Clifford, K. Huygen, R. Tascon, D. Lowrie, M. J. Colston, and R. G. Hewinson. 2000. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine 19:1246-1255. [DOI] [PubMed] [Google Scholar]
- 61.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whipple, D. L., C. A. Bolin, A. J. Davis, J. L. Jarnagin, D. C. Johnson, R. S. Nabors, J. B. Payeur, D. A. Saari, A. J. Wilson, and M. M. Wolf. 1995. Comparison of the sensitivity of the caudal fold skin test and a commercial gamma-interferon assay for diagnosis of bovine tuberculosis. Am. J. Vet. Res. 56:415-419. [PubMed] [Google Scholar]
- 63.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]
- 64.Yam, W. C., M. L. Lung, and M. H. Ng. 1992. Evaluation and optimization of a latex agglutination assay for detection of cholera toxin and Escherichia coli heat-labile toxin. J. Clin. Microbiol. 30:2518-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto, A., M. Nakayama, M. Tashiro, T. Ogawa, and I. Kurane. 2000. Hydroxyapatite-coated nylon beads as a new reagent to develop a particle agglutination assay system for detecting Japanese encephalitis virus-specific human antibodies. J. Clin. Virol. 19:195-204. [DOI] [PubMed] [Google Scholar]