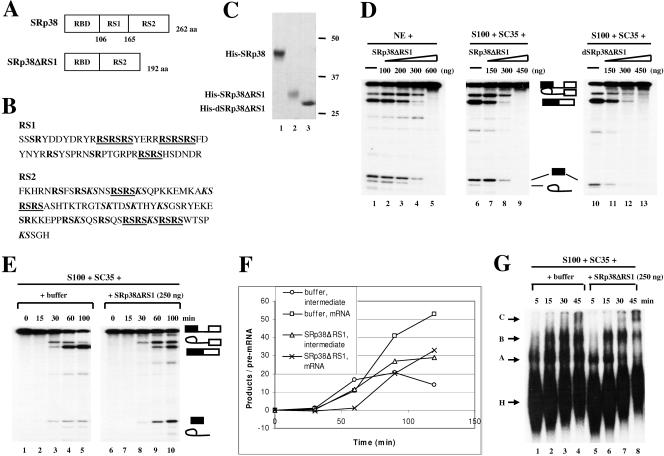
FIG. 4.
Characterization of an internal RS1 domain deletion mutant of SRp38. (A) Diagrams of SRp38 and an internal RS1 domain deletion mutant of SRp38 (SRp38ΔRS1). (B) Sequences of RS1 and RS2 subdomains of SRp38. RS dipeptides are shown in bold, and RS tetra- or hexapeptides are shown in bold and underlined. KS dipeptides are shown in bold italics. (C) Coomassie blue-stained SDS gel of the baculovirus-produced recombinant His-tagged proteins diagrammed in panel A. Dephosphorylated SRp38ΔRS1 (dSRp38ΔRS1, lane 3) was prepared from phosphorylated SRp38ΔRS1 (lane 2) by treatment with CIP and repurification. (D) SRp38ΔRS1 inhibits the second step of splicing. Increasing amounts of SRp38ΔRS1 (lanes 2 to 5 and lanes 7 to 9) or dSRp38ΔRS1 (lanes 11 to 13) were added to in vitro splicing reaction mixtures containing β-globin pre-mRNA and nuclear extract (NE) or S100 extract plus SC35. (E) Time course of β-globin pre-mRNA splicing in S100 extract complemented with 250 ng of SC35 in the presence (lanes 6 to 10) or absence (lanes 1 to 5) of 250 ng of SRp38ΔRS1. (F) Quantitative analysis of β-globin pre-mRNA spliced product (mRNA) and splicing intermediate (lariat/exon2) shown in panel E. (G) SRp38ΔRS1 does not affect spliceosome assembly. Spliceosome assembly was performed with (lanes 5 to 8) or without (lanes 1 to 4) SRp38ΔRS1. Spliceosome complexes were analyzed by 4% native polyacrylamide gel electrophoresis and autoradiography.