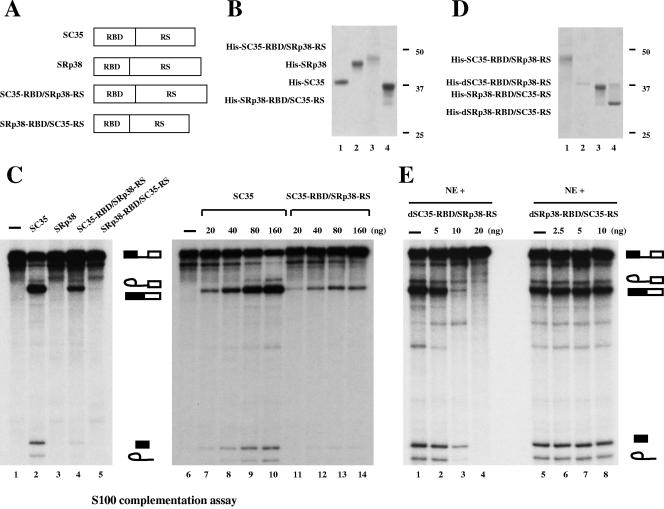
FIG. 6.
Functional domains of SRp38. (A) Diagrams of SC35, SRp38, and domain-swap mutants of these two proteins (RBD of SC35 and RS domain of SRp38 [SC35-RBD/SRp38-RS] and RBD of SRp38 and RS domain of SC35 [SRp38-RBD/SC35-RS]). (B) Coomassie blue-stained SDS gel of the baculovirus-produced recombinant His-tagged proteins diagrammed in panel A. Lane 1, SC35; lane 2, SRp38; lane 3, SC35-RBD/SRp38-RS; lane 4, SRp38-RBD/SC35-RS. (C) SC35-RBD/SRp38-RS weakly activates in vitro splicing of β-globin pre-mRNA splicing in S100 extract. One hundred nanograms of recombinant protein was added to the S100 assay mixtures in lanes 2 to 5, and 20 to 160 ng of protein was used for the assays shown in lanes 7 to 14. (D) Dephosphorylated proteins used for panel E. Dephosphorylated SC35-RBD/SRp38-RS protein (dSC35-RBD/SRp38-RS, lane 2) was prepared from phosphorylated SC35-RBD/SRp38-RS (lane 1) by treatment with CIP and repurification. Dephosphorylated SRp38-RBD/SC35-RS (dSRp38-RBD/SC35-RS, lane 4) was prepared from SRp38-RBD/SC35-RS (lane 3) in the same way as dSC35-RBD/SRp38-RS. (E) The RS domain of SRp38 functions as a modular repression domain. Five to 20 ng of dSC35-RBD/SRp38-RS (lanes 2 to 4) or 2.5 to 10 ng of dSRp38-RBD/SC35-RS (lanes 6 to 8) was added to in vitro splicing reaction mixtures containing β-globin pre-mRNA with nuclear extract (NE).