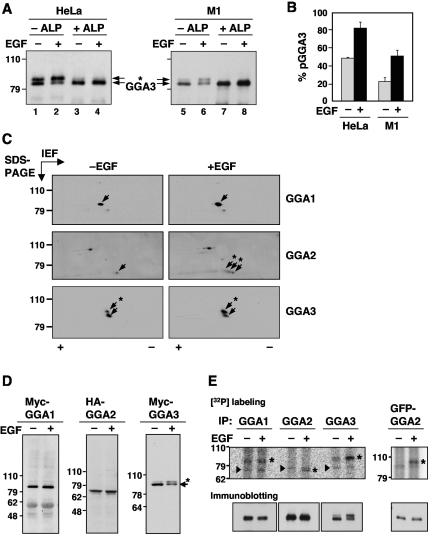
FIG. 1.
EGF-induced phosphorylation of GGA2 and GGA3. (A) Endogenous GGA3 was immunoprecipitated from resting (−EGF, lanes 1, 3, 5, and 7) or EGF-stimulated (+EGF, lanes 2, 4, 6, and 8) HeLa (lanes 1 to 4) or M1 cells (lanes 5 to 8). Immunoprecipitates were incubated in the absence (−ALP, lanes 1, 2, 5, and 6) or presence of alkaline phosphatase (+ALP, lanes 3, 4, 7, and 8), and GGA3 was analyzed by one-dimensional SDS-PAGE and immunoblotting. (B) Quantification of phosphorylated GGA3 (pGGA3) relative to total GGA3 in resting (−EGF) and EGF-stimulated (+EGF) HeLa or M1 cells analyzed as in panel A. Values are the mean ± SD of four independent determinations. (C) M1 fibroblasts were serum-starved for 5 h and incubated for 10 min in the absence (−EGF) or presence (+EGF) of 10 nM EGF, and cell extracts were subjected to two-dimensional isoelectric focusing/SDS-PAGE. Endogenous GGA proteins were detected by immunoblotting. Arrows point to the different GGA species. The symbols + and - indicate the basic and acidic ends, respectively, of the isoelectric focusing. (D) Myc-GGA1, HA-GGA2, and Myc-GGA3 expressed by transfection into resting or EGF-stimulated M1 fibroblasts were analyzed by immunoblotting with antibodies to the epitope tags as in panel A. (E) Untransfected (left panels) or GFP-GGA2-transfected (right panel) M1 fibroblasts were metabolically labeled with 0.5 mCi/ml [32P]orthophosphate for 2 h and incubated in the absence or presence of 10 nM EGF for 10 min. Each GGA was immunoprecipitated with a specific antibody (or anti-GFP antibody for GFP-GGA2) and subjected to one-dimensional SDS-PAGE. Asterisks indicate the GGA proteins, and arrowheads indicate nonspecific bands seen in all lanes. Numbers on the left indicate the positions of molecular mass markers (in kilodaltons), and asterisks in panels A, C, and D indicate the position of phosphorylated GGA3 (throughout the paper, “phosphorylated GGA3” and “pGGA3” are used to denote the more slowly migrating species that increases in intensity upon EGF treatment; the faster migrating species may have other phosphorylated residues that do not affect its electrophoretic mobility).