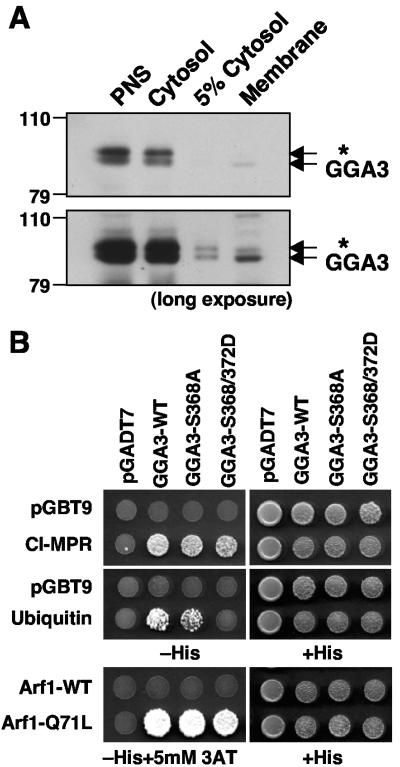
FIG. 8.
Phosphorylation of GGA3 on S368 decreases its recruitment to membranes and binding to ubiquitin. (A) Postnuclear supernatant (PNS), cytosol, and membrane fractions from HeLa cells, prepared as described in Materials and Methods, were subjected to SDS-PAGE and immunoblotting with anti-GGA3 antibody. Short (upper panel) and long (lower panel) exposures of the same blot are shown. (B) Yeast two-hybrid analysis of the binding of GGA3 constructs to the cytosolic tail of the CI-MPR, ubiquitin, wild-type Arf1, and the constitutively activated Arf1-Q71L mutant. pGBT9 and pGADT7 represent “empty” plasmid controls. Cotransformed yeast strains were grown on plates without histidine (−His) or with histidine (+His), and in some cases in the presence of 5 mM 3-aminotriazole (3AT), as needed to suppress nonspecific growth.