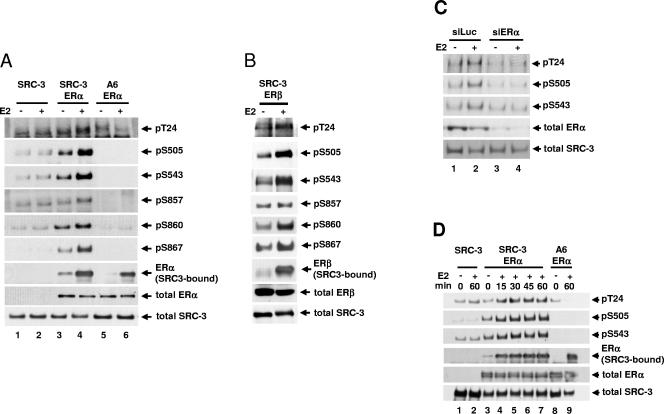
FIG. 2.
Estradiol-induced SRC-3 phosphorylation requires the presence of ER. (A) HEK293T cells were transfected with Flag-tagged SRC-3 (SRC-3) or its phosphorylation mutant (A6), in the absence or presence of ERα. Forty-eight hours later, 10 nM E2 (+) or 0.01% ethanol vehicle (−) was added for 1 h. Cells were then lysed, and Flag-SRC-3 was immunoprecipitated by anti-Flag antibody and separated by SDS-PAGE. Immunoblots were probed with antibodies against each of the six SRC-3 phosphorylation sites (rows 1 to 6). The amount of SRC-3-bound ERα was detected by probing with an anti-ERα antibody (row 7). The total amounts of ERα and SRC-3 in the lysates were determined by immunoblotting with an anti-ERα and an anti-SRC-3 antibody (rows 8 and 9, respectively). (B) Cells were transfected with plasmids for wild-type SRC-3 and ERβ, and the level of SRC-3 phosphorylated at each of the six sites was assessed as described above. (C) siRNA against ERα (siERα) or against luciferase (siLuc) was transfected into MCF-7 cells. Cells were treated as described above. Cells were then lysed, and endogenous SRC-3 was immunoprecipitated using an anti-SRC-3 antibody and separated by SDS-PAGE. The levels of SRC-3 phosphorylated at T24, S505, and S543 were assessed. (D) Cells were transfected as in panel A and treated with 10 nM E2 (+) or 0.01% ethanol (−) for various periods of time (min). The level of SRC-3 phosphorylated at T24, S505, and S543 and the amount of SRC-3-bound ERα were assessed. Shown in the bottom two rows of all the panels are total cell lysates separated by SDS-PAGE and probed with the anti-ERα, anti-ERβ, or anti-SRC-3 antibodies to assess the total cellular levels of ERα, ERβ, and SRC-3, respectively.