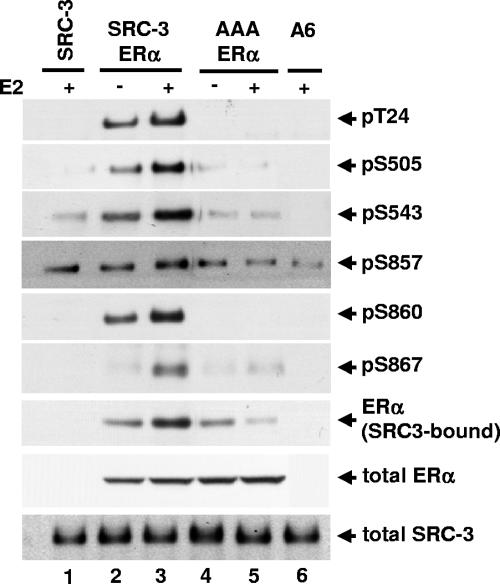
FIG. 6.
The receptor-interacting domain of SRC-3 is required for estradiol-induced SRC-3 phosphorylation. HEK293T cells were transfected with either wild-type Flag-SRC-3 (SRC-3) or SRC-3 mutant (AAA) having all three LXXLL motifs in the receptor-interacting domain mutated to LXXAA, together with wild-type ERα. Forty-eight hours posttransfection, vehicle or E2 (10 nM) was added for 1 h. Cells were then lysed, and Flag-tagged SRC-3 was immunoprecipitated by anti-Flag antibody and separated by SDS-PAGE. The level of SRC-3 phosphorylated at each of the six phosphorylation sites was assessed by immunoblotting with each SRC-3 phosphorylation state-specific antibody (rows 1 to 6). The level of SRC-3-bound ERα was determined by immunoblotting with an anti-ERα antibody (row 7). Total cellular levels of wild-type SRC-3 and its mutant and ERα were also determined by immunoblotting total cell lysates separated by SDS-PAGE with appropriate antibodies (rows 8 and 9).