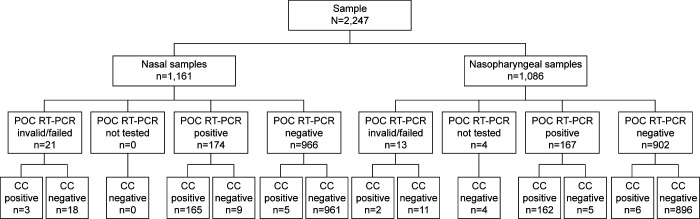
Fig 1.
Detection of SARS-CoV-2 using the POC SARS-CoV-2 & Influenza A/B test and the composite comparator (prospective and retrospective, symptomatic and asymptomatic subjects). The composite comparator comprises the 6800/8800 SARS-CoV-2 & Influenza A/B test, 6800/8800 SARS-CoV-2 test, and Hologic Aptima SARS-CoV-2 Assay; the POC RT-PCR test is the POC SARS-CoV-2 & Influenza A/B test. The Cobas SARS-CoV-2 & Influenza A/B nucleic acid test for use on the Cobas Liat system is here referred to as the POC SARS-CoV-2 & Influenza A/B test. The Cobas SARS-CoV-2 & Influenza A/B qualitative assay for use on the Cobas 6800/8800 systems is here referred to as the 6800/8800 SARS-CoV-2 & Influenza A/B test. Cobas SARS-CoV-2 qualitative assay for use on the Cobas 6800/8800 systems is here referred to as the 6800/8800 SARS-CoV-2 test. Abbreviations: CC, composite comparator; POC RT-PCR, point-of-care RT-PCR.