ABSTRACT
In recent years, high pathogenicity avian influenza viruses (HPAIVs) have spread among wild, captive, and domestic birds, as well as mammals. Beyond the resulting economic and ecological losses, spillover into mammals has raised concerns about a potential pandemic. Viral tropism refers to the spectrum of host species, organs, and cells susceptible and permissive to viral infection. It is a potent driver of infection dynamics and shedding patterns, which presents important variations both between and within hosts: in poultry, HPAIV leads to systemic endothelial infection in domestic chickens, whereas neurological and selective epithelial infections are observed in domestic ducks. In mammals, infection can result in respiratory and neurological disease, but the recent outbreaks in domestic dairy cows highlighted a unique and remarkable adaptation to the mammary gland prone to viral shedding in milk. The present review explores viral tropism of HPAIV across recent spillover from birds to mammals and discusses its critical involvement in viral ecology, requiring the constant surveillance and adaptation of control measures.
KEYWORDS: viral tropism, avian influenza, host adaptation, pathogenesis
INTRODUCTION
Viral tropism can be defined as all living units (i.e., host, organ, tissue, and cellular niches) in which the interaction with a virus leads to functional alteration and eventually perceptible structural alterations. As an information input within a susceptible living unit, viral tropism represents a change in the host activity that supports the completion of viral replication and spread. Viral tropism manifests heterogeneously across living units, creating a dynamic mosaic of susceptible hosts, organs, and cells that drives viral evolution, pathogenesis, and diffusion. Consequently, understanding the viral and host determinants influencing tropism can help in understanding viral ecology, episodic resurgence, and spillover that are raising concerns in terms of pandemic risk.
A BROAD SPECTRUM OF AVIAN HOSTS
Waterbirds represent the natural reservoir of avian influenza viruses (AIVs), including members of Anseriformes (for instance, Anatidae such as ducks, geese, and swan) and Charadriiformes (for instance, Laridae and Scolopacidae such as gulls, terns, and shorebirds) orders (1). The combination of high intestinal permissiveness and low clinical susceptibility makes wild Anseriformes prone to subclinical infection, AIV shedding, circulation, and environmental diffusion (2–4). Due to migratory patterns, they can spread AIVs over long distances (5). Although AIVs are usually restricted to a few species, A/goose/Guangdong/1/1996-like (Gs/Gd) H5 high pathogenicity avian influenza virus (HPAIV) lineage has been detected in a wide range of wild bird species (6, 7). Following the introduction of AIV into poultry farms, most often by wild birds, both gallinaceous and non-gallinaceous birds can be infected. Domestic Galliformes, including chickens, guinea fowls, turkeys, and quails, are clinically susceptible to HPAIV infection (8–11). In contrast, domestic Anseriformes, such as ducks and geese, display variable clinical outcomes but are considered highly susceptible and shedding species over the course of infection (9, 12). Before 2002, HPAIVs were less pathogenic for domestic Anseriformes, which remained mainly asymptomatic. Since then, Gs/Gd H5 HPAIVs have spread, diversified, and resulted in overall increased pathogenicity and marked neurovirulence in domestic Anseriformes (13, 14). Among ratites, ostriches can be infected with both low pathogenicity AIV (LPAIV) and HPAIV (15, 16). In addition to poultry, domestic Columbiformes, such as racing pigeons, can be infected with H5 HPAIV, leading to mild disease and excretion (17, 18). Indirect transmission can be the result of environmental diffusion and bridging hosts facilitating the introduction of AIV into poultry sites (19–21). Synanthropic wild fauna include a wide range of interacting commensal birds, some of which have been reported to interact with domestic ducks (20, 21). Experimentally, infection of crows and European starlings with LPAIVs or HPAIVs leads to infection, shedding, and seroconversion (22–24). Additionally, birds of prey and scavengers are at risk of exposure through the ingestion of infected prey and carcasses (22, 25, 26).
Pathology benefits from the accessibility of infected carcasses to investigate viral tropism in both spontaneous and experimental infected birds. Clinical and pathological findings following HPAIV infection result from various factors, including host (e.g., species, breed, and age), viral strain, inoculum (e.g., inoculation titer and route), co-morbidities, and environmental or experimental settings (9, 11–14, 27). In Galliformes, HPAIV infection leads to multi-visceral failure shock as a result of systemic endotheliitis and disseminated intravascular coagulation (11). Grossly, this acute process results in vascular changes (hyperemia and/or cyanosis), subcutaneous edema, and hemorrhages observed in apteric regions of the integument (i.e., wattle, comb, and unfeathered skin), as well as organomegaly, parenchymal necrosis, congestion, edema, and/or hemorrhage (for instance, in the lung, pancreas, and spleen) (11). Necro-inflammatory foci and vasculitis are indeed widespread in the whole body due to the systemic nature of HPAIV endotheliotropism in those susceptible birds (Fig. 1) (9, 11). In contrast with Galliformes, endothelial tropism is limited in ducks (28–30). HPAIV can, however, spread hematogenously, leading to multicentric necrotizing and non-suppurative inflammation. In general, a pathological triad can be observed, including encephalitis, pancreatitis, and myocarditis (Fig. 1). Microscopically, lesions and viral antigens can be detected among viscera and integument (i.e., growing feathers) with prominent but selective tissue tropism for parenchymal and lining epithelium (9, 11, 31, 32).
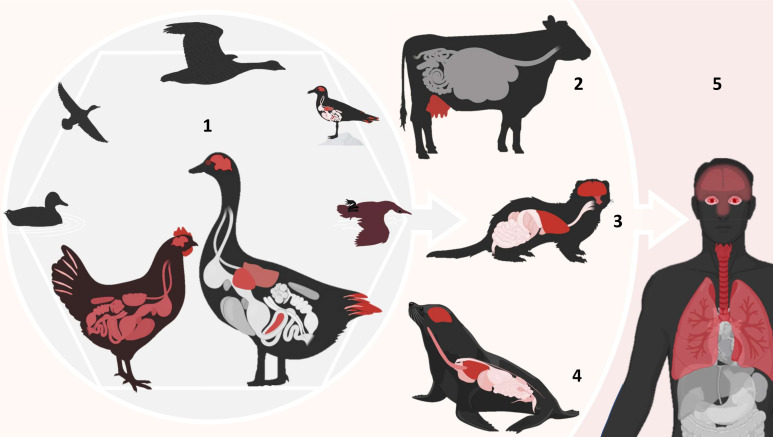
Fig 1.
Selective and host-dependent tissue tropism of H5 HPAIVs from birds to mammals. Organs highlighted in red indicate sites of active viral replication and associated lesions. (1) In domestic Anseriformes, such as ducks, Gs/Gd H5 HPAI infection leads to systemic infection that can progress to neurological disease over the course of infection. Tissue pantropism is selective, including marked neurotropism, visceral tropism, and feather epitheliotropism. The infection course in chickens is shorter due to prominent systemic endotheliotropism resulting in systemic fatal acute disease and widespread viral replication. In addition, wild waterbirds carry and spread Gs/Gd H5 HPAIVs, with variable clinical outcomes ranging from asymptomatic to death. (2) In domestic dairy cows, H5 HPAIV presents a remarkable selective tropism for mammary epithelium leading to diffusion into milk. In carnivorous terrestrial (3) and marine (4) mammals, infection is characterized by a marked tropism for respiratory and nervous tissues. (5) In humans, localized forms of Gs/Gd H5 HPAI infection include conjunctivitis as reported in the dairy cow outbreaks in the United States, while severe forms were associated with prominent respiratory tropism and sometimes neurotropism.
A PANZOOTIC AMONG MAMMALS
H5 HPAIV infection has been reported with increased frequency over the past years in a variety of mammal species, ranging from asymptomatic to mild disease or deadly infections (33–35). Marine and terrestrial carnivorous animals were mostly reported, and infection has been associated with scavenging activity and close contact with infected birds (36, 37). H5 HPAIV infection in marine mammals was reported in sea lions, bottlenose dolphins, harbor seals, and grey seals (35, 38–40). Affected terrestrial mammals included several species of bears, foxes, skunk, mink, mountain lion, cats, and bush dogs (33, 34, 41–48). Most of them were not associated with evidence of mammals-to-mammal transmission, although it was suggested for both terrestrial and marine mammals (34, 44, 49). In naturally dead mammals infected with H5 HPAIV, lesions included non-suppurative necrotizing encephalitis, interstitial pneumonia, and vasculitis (Fig. 1) (33, 40, 50, 51). Neurovirulence is a major feature of HPAIV infection in mammals. Viral entry into the central nervous system can occur through hematogenous spread, or ascending neuroinvasion through cranial nerves, such as olfactory nerves, as has been suggested in carnivorous mammals (52–54). A most recent outcome of this adaptation of avian AIVs to mammals is represented by dairy cows, in which viral replication is confined to the mammary epithelium (Fig. 1), causing necrotizing mastitis during the acute stage of infection (55). This unexpected tropism for the mammary epithelium seems to be largely driven by its avian-like sialic acids (SAs) profile and abundance, including α2-3 SAs in alveolar gland and intralobular ducts (56). Other herbivorous animals, such as horses in Mongolia, were later reported to be infected through serological study (57).
TOWARDS AN INCREASED PANDEMIC RISK
In humans, HPAIV infection was generally associated with close exposure to contaminated animals and carcasses of birds. Eight hundred sixty-eight cases of H5 HPAIV infection were reported over the period 2003–2023 with an overall lethality of 52% (58). Clinical signs included fever, cough, respiratory distress, pneumonia, and, sometimes, neurological signs (59, 60). A pathological investigation conducted on two adults has revealed viral replication within the trachea, alveolar pneumocytes of the lung, brain, small intestine, placenta, and fetus as well as in circulating mononuclear cells, consistent with systemic spread (59). The increased circulation of clade 2.3.4.4b H5N1 viruses in recent years has led to more frequent spillover events, with numerous human cases, particularly in the United States, raising the threat of an emerging virus with pandemic potential (61). Exposure risk is highly dependent on viral tropism that drives viral shedding, as demonstrated by the remarkable epithelial tropism for mammary glands in dairy cows (55). As a consequence, almost all H5N1-infected dairy farm workers, who were exposed to sick cows, developed conjunctivitis (62, 63). In particular, the virus isolated from a farmer’s conjunctiva caused severe infection and lethality in the ferret model, underlining the virus’s ability to adapt to mammals (64). If such infections remained localized, severe cases with the development of severe pneumonia and acute respiratory distress were sporadically and recently reported in Canada and the United States (Fig. 1) (65–67).
MAJOR DETERMINANTS OF AVIAN INFLUENZA VIRUS TROPISM
Once a host is infected, active viral replication leads to the production of an abundant viral progeny that may exhibit genomic variations: a viral quasi-species (68). This phenomenon can arise from various processes, including replicative mutagenesis promoted by error-prone polymerases, recombination, and genome segment reassortments. Viral quasi-species undergo competition, leading to the selection of the most adapted variants in the given environment (69). This can promote increased viral fitness, immune escape, increased virulence, host adaptation, and jump (69, 70). Consequently, replicative mutagenesis and quasi-species genomic mixing represent a driving force of viral tropism, which is governed by a multifactorial set of determinants acting at different stages of the viral life cycle.
One of the earliest barriers is viral entry, which depends on the interaction between viral hemagglutinin (HA) and host cell surface receptors. HA binds to SAs, which are terminal residues of glycans attached to membrane glycoproteins, glycolipids, or mucus. N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) are the most common types, but there are approximately 50 different types of SAs (71). They are attached to underlying sugars (e.g., galactose) of the oligosaccharide chain through a glycosidic bond that can exist in different conformations, such as α2-3 or α2-6 (72).
These conformations are a potent contributor to AIV host range and tropism. Overall, HAs from avian-origin AIVs preferentially bind glycans harboring terminal α2-3 SAs. In contrast, human influenza viruses preferentially bind glycans with terminal α2-6 (73, 74). The distribution and nature of SAs in hosts depend on and vary according to the host species and the organ. For instance, humans predominantly harbor α2-6 SAs in the upper respiratory tract, while α2-3 SAs are mostly expressed in lower portions in alveolar cells and bronchiolar epithelial cells (75, 76). This determines, in part, the lower susceptibility of humans to avian-origin isolates. Among bird species, sialic acid distribution varies notably: geese and ducks predominantly express α2,3-linked receptors throughout the respiratory tract and show minimal α2,6 expression limited to the colon, whereas species like quail exhibit both α2,3 and α2,6 receptors in both the respiratory and intestinal tracts (77).
To be infectious, AIVs require their HA to be enzymatically cleaved. HAs from LPAIVs possess a monobasic cleavage site activated by trypsin-like proteases whose distribution is limited to the respiratory and digestive tracts of birds. This limits infection to localized epithelial tissues. In contrast, HAs from HPAIVs harbor a multibasic cleavage site that allows activation by ubiquitous furin-like proteases, enabling systemic viral spread and tissue pantropism (78–81). Consequently, the viral multibasic cleavage site is a major contributor to HPAIV virulence, by promoting tissue pantropism, systemic disease, and significant mortality (82).
For a virus to cross the species barrier, the adaptation of HA to new SAs is not enough, because the polymerase activity of avian viruses is generally very limited in mammalian cells (83). This is due to an incompatibility between the viral polymerase complex and specific cellular proteins, in particular those of the acidic leucine-rich nuclear phosphoprotein 32 kDa (ANP32) family (84). Mutations in genes encoding the viral polymerase complex can overcome this barrier, such as the PB2 E627K or PB2 D701N mutations (85, 86). These mutations may appear de novo, or may already be present in the index host, in the form of a minority variant. For example, clade 2.3.4.4b H5N1 viruses carrying the PB2 D701N mutation are highly virulent in ferrets, even at low doses, and the reversion of this mutation reduces both mortality and airborne transmission (87). Once the HA and the polymerase complex have adapted, a constellation of other mutations favoring replication and transmissibility generally emerges later, as the virus spreads from host to host.
Viral tropism and host adaptation rely on a complex interplay of molecular mechanisms beyond receptor binding and polymerase compatibility. Additional determinants include pH-dependent HA-mediated membrane fusion, efficient import of viral ribonucleoproteins (vRNPs) into the host nucleus, evasion of innate immune responses, a functional balance between HA binding and neuraminidase (NA) cleavage activities, and other factors (88–91). An exhaustive description of these determinants was provided by (92). As the virus replicates in a new host, constellations of mutations can emerge, enabling more efficient replication and host adaptation (93).
TROPISM AS A MAJOR FACTOR IN SHEDDING AND DIFFUSION PATHWAYS
In permissive hosts, HPAIVs can cause widespread and systemic replication, often leading to high viral loads and substantial environmental shedding. However, this may be counterbalanced by a shortened excretion period due to rapid disease progression and high mortality. For example, chickens are highly susceptible to HPAIV infection, with lower mean death times (MDTs) compared to more resistant species such as ducks (9, 94, 95). In contrast, species that are less clinically susceptible, such as ducks, often exhibit delayed or absent clinical signs, resulting in longer survival times and extended periods of virus replication prior to or without overt disease. Overall, viral tropism, shaped by the interaction between host species and strain virulence, modulates the infection dynamics and influences the balance between pathogenesis and shedding.
Because of their systemic replication, HPAIVs are detected in a wide range of organs. Thus, in the event of an undetected epizootic on a farm, the probability of the virus being present on carcasses after slaughter is greater for HPAIVs than for LPAIVs. Notably, it was through this contamination route that an H5N1 epizootic occurred in Poland, in cats that were fed with contaminated chicken meat (96). In addition, clade 2.3.4.4b H5 HPAIVs exhibit a marked tropism for the feather epithelium in Anseriformes, which can result in the accumulation of infectious viral particles in feather dust (27, 32). Viral replication within growing feather epithelium leads to fragmentation and the release of virus-laden debris (32). While this route of dissemination is less well characterized than fecal or respiratory shedding, it may represent an additional mechanism of environmental contamination and transmission, particularly in settings with high bird density. Its relative contribution to overall transmission dynamics, compared to more established routes, is still unclear and may vary between host species and contexts. However, due to the potential for feather debris to persist in the environment, this pathway could play a role in prolonged environmental contamination and cross-species exposure, including for mammals and humans.
The recent epizootics in dairy cows in the USA are a perfect illustration of the consequences that changes in tropism can have on public health. By replicating in the mammary epithelium, Gs/Gd H5N1 HPAIVs are excreted in large quantities in milk for days: by drinking raw milk, infectious viral particles could come into direct contact with the digestive mucosa, and possibly even the respiratory mucosa, making the consumption of milk an unprecedented source of contamination (55).
TROPISM AND DIAGNOSTIC METHODS
Similarly, viral tropism conditions the relevance of diagnostic methods. In birds, the standard approach involves collecting oropharyngeal, tracheal, and/or cloacal swabs for real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) or viral isolation, in accordance with international guidelines (97). While not yet validated for routine diagnostics, the collection of dust using wipes rubbed against the walls and equipment of poultry buildings, possibly combined with molecules to limit the inhibition of PCR reactions caused by organic material, has been explored as a complementary surveillance, which may allow earlier detection of the virus (19, 98). However, further studies are needed to clarify its sensitivity and utility in different epidemiological contexts. Because viral tropism is different in mammals, the methods used in birds cannot be systematically transposed. For example, during the widespread H5N1 outbreaks on Finnish fur farms in 2023, some infected animals tested negative by RT-qPCR on oropharyngeal swabs, while the virus was detected in brain tissue upon necropsy (99). This likely reflects a progression from initial respiratory infection to systemic and neuroinvasive disease, which is consistent with the neurotropism frequently observed with clade 2.3.4.4b H5Nx viruses in mammals. In such cases, especially during the later stages of infection, necropsy and examination of internal organs, including the brain, may be necessary for definitive diagnosis. However, respiratory or other standard swab specimens remain essential, particularly in early or acute phases of infection. Finally, in H5N1-infected dairy cows, milk is the most relevant sample for virus detection, as there is little to no replication outside the mammary tissue. In addition to individual sampling, novel diagnostic methods highlight the value of wastewater sampling for the detection and monitoring of viral disease (e.g., COVID-19) among the population (100). Wastewater surveillance of H5N1 HPAIV could reveal overall human exposure to contaminated milk and secondary fecal shedding (101).
CONCLUDING REMARKS
Due to their high genetic diversity and ability to generate new variants, AIVs have a broad host spectrum and episodically across the species barrier. From one species to another, the tissues and cells supporting viral replication can be radically different. Between the feather epitheliotropism in ducks (resulting in the generation of infectious dust), the mammary epitheliotropism in dairy cows (resulting in the excretion of viral particles in milk), and the neurotropism observed in carnivorous mammals (often resulting in an epidemiological dead-end for the virus), control and diagnostic measures, as well as the risk of dissemination, are totally different. Investigating viral tropism in all host species, based on a close collaboration of clinicians, pathologists, and virologists, should therefore be a priority for a better understanding and anticipation of viral emergence.
Contributor Information
Nicolas Gaide, Email: nicolas.gaide@envt.fr.
Vinayaka R. Prasad, Albert Einstein College of Medicine, Bronx, New York, USA
REFERENCES
- 1. Stallknecht DE, Brown JD. 2008. Ecology of avian influenza in wild birds, p 43–58. In Avian Influenza. John Wiley & Sons, Ltd. [Google Scholar]
- 2. Gaidet N, Cappelle J, Takekawa JY, Prosser DJ, Iverson SA, Douglas DC, Perry WM, Mundkur T, Newman SH. 2010. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large‐scale satellite telemetry. Journal of Applied Ecology 47:1147–1157. doi: 10.1111/j.1365-2664.2010.01845.x [DOI] [Google Scholar]
- 3. Tanikawa T, Sakuma S, Yoshida E, Tsunekuni R, Nakayama M, Kobayashi S. 2021. Comparative susceptibility of the common teal (Anas crecca) to infection with high pathogenic avian influenza virus strains isolated in Japan in 2004-2017. Vet Microbiol 263:109266. doi: 10.1016/j.vetmic.2021.109266 [DOI] [PubMed] [Google Scholar]
- 4. van den Brand JMA, Verhagen JH, Veldhuis Kroeze EJB, van de Bildt MWG, Bodewes R, Herfst S, Richard M, Lexmond P, Bestebroer TM, Fouchier RAM, Kuiken T. 2018. Wild ducks excrete highly pathogenic avian influenza virus H5N8 (2014-2015) without clinical or pathological evidence of disease. Emerg Microbes Infect 7:67. doi: 10.1038/s41426-018-0070-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lv X, Li X, Sun H, Li Y, Peng P, Qin S, et al. 2020. Viruses in satellite-tracked wild ducks, Ningxia, China. Emerg Infect Dis 28:1039. doi: 10.3201/eid2805.211580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klaassen M, Wille M. 2023. The plight and role of wild birds in the current bird flu panzootic. Nat Ecol Evol 7:1541–1542. doi: 10.1038/s41559-023-02182-x [DOI] [PubMed] [Google Scholar]
- 7. Xie R, Edwards KM, Wille M, Wei X, Wong S-S, Zanin M, El-Shesheny R, Ducatez M, Poon LLM, Kayali G, Webby RJ, Dhanasekaran V. 2023. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 622:810–817. doi: 10.1038/s41586-023-06631-2 [DOI] [PubMed] [Google Scholar]
- 8. Bertran K, Lee D-H, Pantin-Jackwood MJ, Spackman E, Balzli C, Suarez DL, Swayne DE. 2017. Pathobiology of clade 2.3.4.4 H5Nx high-pathogenicity avian influenza virus infections in minor gallinaceous poultry supports early backyard flock introductions in the western United States in 2014-2015. J Virol 91:e00960-17. doi: 10.1128/JVI.00960-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. James J, Billington E, Warren CJ, De Sliva D, Di Genova C, Airey M, Meyer SM, Lewis T, Peers-Dent J, Thomas SS, Lofts A, Furman N, Nunez A, Slomka MJ, Brown IH, Banyard AC. 2023. Clade 2.3.4.4b H5N1 high pathogenicity avian influenza virus (HPAIV) from the 2021/22 epizootic is highly duck adapted and poorly adapted to chickens. J Gen Virol 104:001852. doi: 10.1099/jgv.0.001852 [DOI] [PubMed] [Google Scholar]
- 10. Pantin-Jackwood MJ, Spackman E, Leyson C, Youk S, Lee SA, Moon LM, Torchetti MK, Killian ML, Lenoch JB, Kapczynski DR, Swayne DE, Suarez DL. 2023. Pathogenicity in chickens and turkeys of a 2021 United States H5N1 highly pathogenic avian influenza clade 2.3.4.4b wild bird virus compared to two previous H5N8 clade 2.3.4.4 viruses. Viruses 15:2273. doi: 10.3390/v15112273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaide N, Lucas M-N, Delpont M, Croville G, Bouwman KM, Papanikolaou A, van der Woude R, Gagarinov IA, Boons G-J, De Vries RP, Volmer R, Teillaud A, Vergne T, Bleuart C, Le Loc’h G, Delverdier M, Guérin J-L. 2022. Pathobiology of highly pathogenic H5 avian influenza viruses in naturally infected Galliformes and Anseriformes in France during winter 2015-2016. Vet Res 53:11. doi: 10.1186/s13567-022-01028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figueroa T, Bessière P, Coggon A, Bouwman KM, van der Woude R, Delverdier M, Verheije MH, de Vries RP, Volmer R. 2020. The microbiota contributes to the control of highly pathogenic H5N9 influenza virus replication in ducks. J Virol 94:e00289-20. doi: 10.1128/JVI.00289-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foret-Lucas C, Figueroa T, Coggon A, Houffschmitt A, Dupré G, Fusade-Boyer M, Guérin J-L, Delverdier M, Bessière P, Volmer R. 2023. In vitro and in vivo characterization of H5N8 high-pathogenicity avian influenza virus neurotropism in ducks and chickens. Microbiol Spectr 11:e04229-22. doi: 10.1128/spectrum.04229-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantin-jackwook MJ, Swayne DE. 2009. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev Sci Tech OIE 28:113–136. doi: 10.20506/rst.28.1.1869 [DOI] [PubMed] [Google Scholar]
- 15. Abolnik C, Ostmann E, Woods M, Wandrag DBR, Grewar J, Roberts L, Olivier AJ. 2021. Experimental infection of ostriches with H7N1 low pathogenic and H5N8 clade 2.3.4.4B highly pathogenic influenza A viruses. Vet Microbiol 263:109251. doi: 10.1016/j.vetmic.2021.109251 [DOI] [PubMed] [Google Scholar]
- 16. Elsayed HS, Adel A, Alshaya DS, Safhi FA, jalal AS, Elmasry DMA, Selim K, Erfan AA, Eid S, Selim S, El-Saadony MT, Shahein M. 2022. First isolation of influenza a subtype H5N8 in ostrich: pathological and genetic characterization. Poult Sci 101:102156. doi: 10.1016/j.psj.2022.102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perkins LEL, Swayne DE. 2002. Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Dis 46:53–63. doi: 10.1637/0005-2086(2002)046[0053:POAHKO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 18. Abolnik C, Stutchbury S, Hartman MJ. 2018. Experimental infection of racing pigeons (Columba livia domestica) with highly pathogenic clade 2.3.4.4 sub-group B H5N8 avian influenza virus. Vet Microbiol 227:127–132. doi: 10.1016/j.vetmic.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 19. Filaire F, Lebre L, Foret-Lucas C, Vergne T, Daniel P, Lelièvre A, de Barros A, Jbenyeni A, Bolon P, Paul M, Croville G, Guérin J-L. 2022. Highly pathogenic avian influenza A(H5N8) clade 2.3.4.4b virus in dust samples from poultry farms, France, 2021. Emerg Infect Dis 28:1446–1450. doi: 10.3201/eid2807.212247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graziosi G, Lupini C, Favera FD, Martini G, Dosa G, Trevisani G, Garavini G, Mannelli A, Catelli E. 2024. Characterizing the domestic-wild bird interface through camera traps in an area at risk for avian influenza introduction in Northern Italy. Poult Sci 103:103892. doi: 10.1016/j.psj.2024.103892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Gall-Ladevèze C, Guinat C, Fievet P, Vollot B, Guérin J-L, Cappelle J, Le Loc’h G. 2022. Quantification and characterisation of commensal wild birds and their interactions with domestic ducks on a free-range farm in southwest France. Sci Rep 12:9764. doi: 10.1038/s41598-022-13846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verma AK, Kumar M, Murugkar HV, Nagarajan S, Tosh C, Namdeo P, Singh R, Mishra S, Senthilkumar D, Singh VP, Sanyal A. 2023. Highly pathogenic avian influenza (H5N1) infection in crows through ingestion of infected crow carcasses. Microb Pathog 183:106330. doi: 10.1016/j.micpath.2023.106330 [DOI] [PubMed] [Google Scholar]
- 23. Ellis JW, Root JJ, McCurdy LM, Bentler KT, Barrett NL, VanDalen KK, Dirsmith KL, Shriner SA. 2021. Avian influenza A virus susceptibility, infection, transmission, and antibody kinetics in European starlings. PLoS Pathog 17:e1009879. doi: 10.1371/journal.ppat.1009879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reid SM, Byrne AMP, Lean FZX, Ross CS, Pascu A, Hepple R, Dominguez M, Frost S, Coward VJ, Núñez A, James J, Stephan L, Aegerter JN, Brown IH, Banyard AC. 2024. A multi-species, multi-pathogen avian viral disease outbreak event: investigating potential for virus transmission at the wild bird - poultry interface. Emerg Microbes Infect 13:2348521. doi: 10.1080/22221751.2024.2348521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duriez O, Sassi Y, Le Gall-Ladevèze C, Giraud L, Straughan R, Dauverné L, Terras A, Boulinier T, Choquet R, Van De Wiele A, Hirschinger J, Guérin J-L, Le Loc’h G. 2023. Highly pathogenic avian influenza affects vultures’ movements and breeding output. Curr Biol 33:3766–3774. doi: 10.1016/j.cub.2023.07.061 [DOI] [PubMed] [Google Scholar]
- 26. Lean FZX, Falchieri M, Furman N, Tyler G, Robinson C, Holmes P, Reid SM, Banyard AC, Brown IH, Man C, Núñez A. 2024. Highly pathogenic avian influenza virus H5N1 infection in skua and gulls in the United Kingdom, 2022. Vet Pathol 61:421–431. doi: 10.1177/03009858231217224 [DOI] [PubMed] [Google Scholar]
- 27. Gaide N, Foret-Lucas C, Figueroa T, Vergne T, Lucas M-N, Robertet L, Souvestre M, Croville G, Le Loc’h G, Delverdier M, Guérin J-L. 2021. Viral tropism and detection of clade 2.3.4.4b H5N8 highly pathogenic avian influenza viruses in feathers of ducks and geese. Sci Rep 11:5928. doi: 10.1038/s41598-021-85109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Bruin ACM, Spronken MI, Bestebroer TM, Fouchier RAM, Richard M. 2022. Reduced replication of highly pathogenic avian influenza virus in duck endothelial cells compared to chicken endothelial cells is associated with stronger antiviral responses. Viruses 14:165. doi: 10.3390/v14010165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bertran K, Swayne DE, Pantin-Jackwood MJ, Kapczynski DR, Spackman E, Suarez DL. 2016. Lack of chicken adaptation of newly emergent Eurasian H5N8 and reassortant H5N2 high pathogenicity avian influenza viruses in the U.S. is consistent with restricted poultry outbreaks in the Pacific flyway during 2014-2015. Virology (Auckland) 494:190–197. doi: 10.1016/j.virol.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 30. Bae Y-J, Lee S-B, Min K-C, Mo J-S, Jeon E-O, Koo B-S, Kwon H-I, Choi YK, Kim J-J, Kim J-N, Mo I-P. 2015. Pathological evaluation of natural cases of a highly pathogenic avian influenza virus, subtype H5N8, in broiler breeders and commercial layers in South Korea. Avian Dis 59:175–182. doi: 10.1637/10921-081914-case [DOI] [PubMed] [Google Scholar]
- 31. Dinev I, Zarkov I, Goujgoulova GV, Stoimenov GM, Georgiev G, Kanakov D. 2020. Pathologic evaluation of influenza A H5N8 infection outbreaks in mule ducks in Bulgaria. Avian Dis 64:203–209. doi: 10.1637/0005-2086-64.2.203 [DOI] [PubMed] [Google Scholar]
- 32. Gaide N, Filaire F, Bertran K, Crispo M, Dirat M, Secula A, Foret-Lucas C, Payré B, Perlas A, Cantero G, Majó N, Soubies S, Guérin J-L. 2023. The feather epithelium contributes to the dissemination and ecology of clade 2.3.4.4b H5 high pathogenicity avian influenza viruses in ducks. Emerg Microbes Infect 12:2272644. doi: 10.1080/22221751.2023.2272644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bessière P, Gaide N, Croville G, Crispo M, Fusade-Boyer M, Abou Monsef Y, et al. 2024. High pathogenicity avian influenza A (H5N1) clade 2.3.4.4b virus infection in a captive Tibetan black bear. Microbiol Spectr 12:e03736-23. doi: 10.1128/spectrum.03736-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, Siepker C, Mainenti M, Li G, Thompson AC, Gorden PJ, Plummer PJ, Main R. 2024. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis 30:1335–1343. doi: 10.3201/eid3007.240508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ulloa M, Fernández A, Ariyama N, Colom-Rivero A, Rivera C, Nuñez P, Sanhueza P, Johow M, Araya H, Torres JC, Gomez P, Muñoz G, Agüero B, Alegría R, Medina R, Neira V, Sierra E. 2023. Mass mortality event in South American sea lions (Otaria flavescens) correlated to highly pathogenic avian influenza (HPAI) H5N1 outbreak in Chile. Vet Q 43:1–10. doi: 10.1080/01652176.2023.2265173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plaza PI, Gamarra-Toledo V, Euguí JR, Lambertucci SA. 2024. Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerg Infect Dis 30:444–452. doi: 10.3201/eid3003.231098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomás G, Marandino A, Panzera Y, Rodríguez S, Wallau GL, Dezordi FZ, Pérez R, Bassetti L, Negro R, Williman J, Uriarte V, Grazioli F, Leizagoyen C, Riverón S, Coronel J, Bello S, Páez E, Lima M, Méndez V, Pérez R. 2024. Highly pathogenic avian influenza H5N1 virus infections in pinnipeds and seabirds in Uruguay: implications for bird–mammal transmission in South America. Virus Evol 10. doi: 10.1093/ve/veae031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leguia M, Garcia-Glaessner A, Muñoz-Saavedra B, Juarez D, Barrera P, Calvo-Mac C, Jara J, Silva W, Ploog K, Amaro, Lady, Colchao-Claux P, Johnson CK, Uhart MM, Nelson MI, Lescano J. 2023. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun 14:5489. doi: 10.1038/s41467-023-41182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Postel A, King J, Kaiser FK, Kennedy J, Lombardo MS, Reineking W, de le Roi M, Harder T, Pohlmann A, Gerlach T, Rimmelzwaan G, Rohner S, Striewe LC, Gross S, Schick LA, Klink JC, Kramer K, Osterhaus ADME, Beer M, Baumgärtner W, Siebert U, Becher P. 2022. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg Microbes Infect 11:725–729. doi: 10.1080/22221751.2022.2043726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mirolo M, Pohlmann A, Ahrens AK, Kühl B, Rubio-Garcìa A, Kramer K, Meinfelder U, Rosenberger T, Morito HL, Beer M, Ludlow M, Wohlsein P, Baumgärtner W, Harder T, Osterhaus A. 2023. Highly pathogenic avian influenza A virus (HPAIV) H5N1 infection in two European grey seals ( Halichoerus grypus ) with encephalitis . Emerg Microbes Infect 12. doi: 10.1080/22221751.2023.2257810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jakobek BT, Berhane Y, Nadeau M-S, Embury-Hyatt C, Lung O, Xu W, Lair S. 2023. Influenza A(H5N1) virus infections in 2 free-ranging black bears (Ursus americanus), Quebec, Canada. Emerg Infect Dis 29:2145–2149. doi: 10.3201/eid2910.230548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elsmo EJ, Wünschmann A, Beckmen KB, Broughton-Neiswanger LE, Buckles EL, Ellis J, Fitzgerald SD, Gerlach R, Hawkins S, Ip HS, et al. 2023. Highly pathogenic avian influenza A(H5N1) virus clade 2.3.4.4b infections in wild terrestrial mammals, United States, 2022. Emerg Infect Dis 29:2451–2460. doi: 10.3201/eid2912.230464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bordes L, Vreman S, Heutink R, Roose M, Venema S, Pritz-Verschuren SBE, Rijks JM, Gonzales JL, Germeraad EA, Engelsma M, Beerens N. 2023. Highly pathogenic avian influenza H5N1 virus infections in wild red foxes (Vulpes vulpes) show neurotropism and adaptive virus mutations. Microbiol Spectr 11:e0286722. doi: 10.1128/spectrum.02867-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agüero M, Monne I, Sánchez A, Zecchin B, Fusaro A, Ruano MJ, Del Valle Arrojo M, Fernández-Antonio R, Souto AM, Tordable P, Cañás J, Bonfante F, Giussani E, Terregino C, Orejas JJ. 2023. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill 28:2300001. doi: 10.2807/1560-7917.ES.2023.28.3.2300001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Briand F-X, Souchaud F, Pierre I, Beven V, Hirchaud E, Hérault F, Planel R, Rigaudeau A, Bernard-Stoecklin S, Van der Werf S, Lina B, Gerbier G, Eterradossi N, Schmitz A, Niqueux E, Grasland B. 2023. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in domestic cat, France, 2022. Emerg Infect Dis 29:1696–1698. doi: 10.3201/eid2908.230188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erdelyan CNG, Kandeil A, Signore AV, Jones MEB, Vogel P, Andreev K, Bøe CA, Gjerset B, Alkie TN, Yason C, Hisanaga T, Sullivan D, Lung O, Bourque L, Ayilara I, Pama L, Jeevan T, Franks J, Jones JC, Seiler JP, Miller L, Mubareka S, Webby RJ, Berhane Y. 2024. Multiple transatlantic incursions of highly pathogenic avian influenza clade 2.3.4.4b A(H5N5) virus into North America and spillover to mammals. Cell Rep 43:114479. doi: 10.1016/j.celrep.2024.114479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Floyd T, Banyard AC, Lean FZX, Byrne AMP, Fullick E, Whittard E, Mollett BC, Bexton S, Swinson V, Macrelli M, Lewis NS, Reid SM, Núñez A, Duff JP, Hansen R, Brown IH. 2021. Encephalitis and death in wild mammals at a rehabilitation center after infection with highly pathogenic avian influenza A(H5N8) virus, United Kingdom. Emerg Infect Dis 27:2856–2863. doi: 10.3201/eid2711.211225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falchieri M, Reid SM, Dastderji A, Cracknell J, Warren CJ, Mollett BC, Peers-Dent J, Schlachter A-LD, Mcginn N, Hepple R, Thomas S, Ridout S, Quayle J, Pizzi R, Núñez A, Byrne AMP, James J, Banyard AC. 2024. Rapid mortality in captive bush dogs (Speothos venaticus) caused by influenza A of avian origin (H5N1) at a wildlife collection in the United Kingdom. Emerg Microbes Infect 13:2361792. doi: 10.1080/22221751.2024.2361792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Uhart MM, Vanstreels RET, Nelson MI, Olivera V, Campagna J, Zavattieri V, Lemey P, Campagna C, Falabella V, Rimondi A. 2024. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nat Commun 15:9516. doi: 10.1038/s41467-024-53766-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chothe SK, Srinivas S, Misra S, Nallipogu NC, Gilbride E, LaBella L, Mukherjee S, Gauthier CH, Pecoraro HL, Webb BT, Pipas JM, Ramasamy S, Kuchipudi SV. 2025. Marked neurotropism and potential adaptation of H5N1 clade 2.3.4.4.b virus in naturally infected domestic cats. Emerging Microbes & Infections 14. doi: 10.1080/22221751.2024.2440498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sillman SJ, Drozd M, Loy D, Harris SP. 2023. Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J Comp Pathol 205:17–23. doi: 10.1016/j.jcpa.2023.07.001 [DOI] [PubMed] [Google Scholar]
- 52. Plourde JR, Pyles JA, Layton RC, Vaughan SE, Tipper JL, Harrod KS. 2012. Neurovirulence of H5N1 infection in ferrets is mediated by multifocal replication in distinct permissive neuronal cell regions. PLoS One 7:e46605. doi: 10.1371/journal.pone.0046605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schrauwen EJA, Herfst S, Leijten LM, van Run P, Bestebroer TM, Linster M, Bodewes R, Kreijtz JHCM, Rimmelzwaan GF, Osterhaus ADME, Fouchier RAM, Kuiken T, van Riel D. 2012. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J Virol 86:3975–3984. doi: 10.1128/JVI.06828-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bauer L, Benavides FFW, Veldhuis Kroeze EJB, de Wit E, van Riel D. 2023. The neuropathogenesis of highly pathogenic avian influenza H5Nx viruses in mammalian species including humans. Trends Neurosci 46:953–970. doi: 10.1016/j.tins.2023.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caserta LC, Frye EA, Butt SL, Laverack M, Nooruzzaman M, Covaleda LM, Thompson AC, Koscielny MP, Cronk B, Johnson A, Kleinhenz K, Edwards EE, Gomez G, Hitchener G, Martins M, Kapczynski DR, Suarez DL, Alexander Morris ER, Hensley T, Beeby JS, Lejeune M, Swinford AK, Elvinger F, Dimitrov KM, Diel DG. 2024. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 634:669–676. doi: 10.1038/s41586-024-07849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nelli RK, Harm TA, Siepker C, Groeltz-Thrush JM, Jones B, Twu N-C, Nenninger AS, Magstadt DR, Burrough ER, Piñeyro PE, Mainenti M, Carnaccini S, Plummer PJ, Bell TM. 2024. Sialic acid receptor specificity in mammary gland of dairy cattle infected with highly pathogenic avian influenza A(H5N1) virus. Emerg Infect Dis 30:1361–1373. doi: 10.3201/eid3007.240689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Damdinjav B, Raveendran S, Mojsiejczuk L, Ankhanbaatar U, Yang J, Sadeyen J-R, Iqbal M, Perez DR, Rajao DS, Park A, Viana M, Murcia PR. 2025. Evidence of influenza A(H5N1) spillover infections in horses, Mongolia. Emerg Infect Dis 31:183–185. doi: 10.3201/eid3101.241266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. World Health Organization . 2023. Cumulative number of confirmed human cases for avian influenza A (H5N1). https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk-assessments/cumulative-number-of--confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-2023.pdf.
- 59. Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Gao Z, Zhang B, McNutt MA, Lu M, Anderson VM, Gong E, Yu ACH, Lipkin WI. 2007. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 370:1137–1145. doi: 10.1016/S0140-6736(07)61515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang L, Liu K, Su Q, Chen X, Wang X, Li Q, Wang W, Mao X, Xu J, Zhou X, Xu Q, Zhou L, Liu X, Zhang P. 2022. Clinical features of the first critical case of acute encephalitis caused by the avian influenza A (H5N6) virus. Emerg Microbes Infect 11:2437–2446. doi: 10.1080/22221751.2022.2122584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garg S, Reinhart K, Couture A, Kniss K, Davis CT, Kirby MK, Murray EL, Zhu S, Kraushaar V, Wadford DA, et al. 2025. Highly pathogenic avian influenza A(H5N1) virus infections in humans. N Engl J Med 392:843–854. doi: 10.1056/NEJMoa2414610 [DOI] [PubMed] [Google Scholar]
- 62. Morse J, Coyle J, Mikesell L, Stoddard B, Eckel S, Weinberg M, Kuo J, Riner D, Margulieux K, Stricklen J, Dover M, Kniss KL, Jang Y, Kirby MK, Frederick JC, Lacek KA, Davis CT, Uyeki TM, Lyon-Callo S, Bagdasarian N. 2024. Influenza A(H5N1) virus infection in two dairy farm workers in Michigan. N Engl J Med 391:963–964. doi: 10.1056/NEJMc2407264 [DOI] [PubMed] [Google Scholar]
- 63. Venkatesan P. 2024. Human cases of avian influenza A(H5) in the USA. Lancet Microbe 5:100978. doi: 10.1016/j.lanmic.2024.100978 [DOI] [PubMed] [Google Scholar]
- 64. Belser JA, Sun X, Pulit-Penaloza JA, Maines TR. 2024. Fatal infection in ferrets after ocular inoculation with highly pathogenic avian influenza A(H5N1). Emerg Infect Dis 30:1484. doi: 10.3201/eid3007.240520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Centers of Disease and Prevention . 2024. CDC confirms first severe case of H5N1 bird flu in the United States. https://www.cdc.gov/media/releases/2024/m1218-h5n1-flu.html.
- 66. Dyer O. 2024. Bird flu: Canadian teenager is critically ill with new genotype. BMJ 387:q2529. doi: 10.1136/bmj.q2529 [DOI] [PubMed] [Google Scholar]
- 67. Jassem AN, Roberts A, Tyson J, Zlosnik JEA, Russell SL, Caleta JM, Eckbo EJ, Gao R, Chestley T, Grant J, Uyeki TM, Prystajecky NA, Himsworth CG, MacBain E, Ranadheera C, Li L, Hoang LMN, Bastien N, Goldfarb DM. 2025. Critical illness in an adolescent with influenza A(H5N1) virus infection. N Engl J Med 392:927–929. doi: 10.1056/NEJMc2415890 [DOI] [PubMed] [Google Scholar]
- 68. Eigen M. 1993. Viral quasispecies. Sci Am 269:42–49. doi: 10.1038/scientificamerican0793-42 [DOI] [PubMed] [Google Scholar]
- 69. Bessière P, Volmer R. 2021. From one to many: the within-host rise of viral variants. PLoS Pathog 17:e1009811. doi: 10.1371/journal.ppat.1009811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sanjuán R. 2021. The social life of viruses. Annu Rev Virol 8:183–199. doi: 10.1146/annurev-virology-091919-071712 [DOI] [PubMed] [Google Scholar]
- 71. Schauer R. 2004. Sialic acids: fascinating sugars in higher animals and man. Zoology (Jena) 107:49–64. doi: 10.1016/j.zool.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 72. Verhagen JH, Eriksson P, Leijten L, Blixt O, Olsen B, Waldenström J, Ellström P, Kuiken T. 2021. Host range of influenza A virus H1 to H16 in eurasian ducks based on tissue and receptor binding studies. J Virol 95:e01873-20. doi: 10.1128/JVI.01873-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hiono T, Okamatsu M, Nishihara S, Takase-Yoden S, Sakoda Y, Kida H. 2014. A chicken influenza virus recognizes fucosylated α2,3 sialoglycan receptors on the epithelial cells lining upper respiratory tracts of chickens. Virology (Auckland) 456–457:131–138. doi: 10.1016/j.virol.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 74. Rogers GN, Paulson JC. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology (Auckl) 127:361–373. doi: 10.1016/0042-6822(83)90150-2 [DOI] [PubMed] [Google Scholar]
- 75. Kumlin U, Olofsson S, Dimock K, Arnberg N. 2008. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Viruses 2:147–154. doi: 10.1111/j.1750-2659.2008.00051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shi Y, Wu Y, Zhang W, Qi J, Gao GF. 2014. Enabling the “host jump”: structural determinants of receptor-binding specificity in influenza A viruses. Nat Rev Microbiol 12:822–831. doi: 10.1038/nrmicro3362 [DOI] [PubMed] [Google Scholar]
- 77. Kimble B, Nieto GR, Perez DR. 2010. Characterization of influenza virus sialic acid receptors in minor poultry species. Virol J 7:365. doi: 10.1186/1743-422X-7-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Horimoto T, Kawaoka Y. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol 3:591–600. doi: 10.1038/nrmicro1208 [DOI] [PubMed] [Google Scholar]
- 79. de Bruin ACM, Spronken MI, Bestebroer TM, Fouchier RAM, Richard M. 2023. Conserved expression and functionality of furin between chickens and ducks as an activating protease of highly pathogenic avian influenza virus hemagglutinins. Microbiol Spectr 11:e04602-22. doi: 10.1128/spectrum.04602-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakayama K. 1991. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem 266:12127–12130. [PubMed] [Google Scholar]
- 81. Thomas G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 3:753–766. doi: 10.1038/nrm934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stieneke-Gröber A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk HD, Garten W. 1992. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J 11:2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gabriel G, Dauber B, Wolff T, Planz O, Klenk HD, Stech J. 2005. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc Natl Acad Sci USA 102:18590–18595. doi: 10.1073/pnas.0507415102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Long JS, Giotis ES, Moncorgé O, Frise R, Mistry B, James J, Morisson M, Iqbal M, Vignal A, Skinner MA, Barclay WS. 2016. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature 529:101–104. doi: 10.1038/nature16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Czudai-Matwich V, Otte A, Matrosovich M, Gabriel G, Klenk HD. 2014. PB2 mutations D701N and S714R promote adaptation of an influenza H5N1 virus to a mammalian host. J Virol 88:8735–8742. doi: 10.1128/JVI.00422-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shinya K, Hamm S, Hatta M, Ito H, Ito T, Kawaoka Y. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology (Auckland) 320:258–266. doi: 10.1016/j.virol.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 87. Restori KH, Septer KM, Field CJ, Patel DR, VanInsberghe D, Raghunathan V, Lowen AC, Sutton TC. 2024. Risk assessment of a highly pathogenic H5N1 influenza virus from mink. Nat Commun 15:4112. doi: 10.1038/s41467-024-48475-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. DuBois RM, Zaraket H, Reddivari M, Heath RJ, White SW, Russell CJ. 2011. Acid stability of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity. PLoS Pathog 7:e1002398. doi: 10.1371/journal.ppat.1002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Naffakh N, Tomoiu A, Rameix-Welti M-A, van der Werf S. 2008. Host restriction of avian influenza viruses at the level of the ribonucleoproteins. Annu Rev Microbiol 62:403–424. doi: 10.1146/annurev.micro.62.081307.162746 [DOI] [PubMed] [Google Scholar]
- 90. Schmolke M, García-Sastre A. 2010. Evasion of innate and adaptive immune responses by influenza A virus. Cell Microbiol 12:873–880. doi: 10.1111/j.1462-5822.2010.01475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gaymard A, Le Briand N, Frobert E, Lina B, Escuret V. 2016. Functional balance between neuraminidase and haemagglutinin in influenza viruses. Clin Microbiol Infect 22:975–983. doi: 10.1016/j.cmi.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 92. Long JS, Mistry B, Haslam SM, Barclay WS. 2019. Host and viral determinants of influenza A virus species specificity. Nat Rev Microbiol 17:67–81. doi: 10.1038/s41579-018-0115-z [DOI] [PubMed] [Google Scholar]
- 93. Taubenberger JK, Kash JC. 2010. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 7:440–451. doi: 10.1016/j.chom.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bessière P, Figueroa T, Coggon A, Foret-Lucas C, Houffschmitt A, Fusade-Boyer M, Dupré G, Guérin J-L, Delverdier M, Volmer R. 2022. Opposite outcomes of the within-host competition between high- and low-pathogenic H5N8 avian influenza viruses in chickens compared to ducks. J Virol 96:e0136621. doi: 10.1128/JVI.01366-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aldous EW, Seekings JM, McNally A, Nili H, Fuller CM, Irvine RM, Alexander DJ, Brown IH. 2010. Infection dynamics of highly pathogenic avian influenza and virulent avian paramyxovirus type 1 viruses in chickens, turkeys and ducks. Avian Pathol 39:265–273. doi: 10.1080/03079457.2010.492825 [DOI] [PubMed] [Google Scholar]
- 96. Rabalski L, Milewska A, Pohlmann A, Gackowska K, Lepionka T, Szczepaniak K, Swiatalska A, Sieminska I, Arent Z, Beer M, Koopmans M, Grzybek M, Pyrc K. 2023. Emergence and potential transmission route of avian influenza A (H5N1) virus in domestic cats in Poland, June 2023. Euro Surveill 28:2300390. doi: 10.2807/1560-7917.ES.2023.28.31.2300390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martin V, Forman A, Lubroth J. 2009. Preparing for highly pathogenic avian influenza. Rome: Food and Agriculture Organization of the United Nations [Google Scholar]
- 98. Bessière P, Hayes B, Filaire F, Lèbre L, Vergne T, Pinson M, Croville G, Guérin J-L. 2023. Optimizing environmental viral surveillance: bovine serum albumin increases RT-qPCR sensitivity for high pathogenicity avian influenza H5Nx virus detection from dust samples. Microbiol Spectr 11:e03055-23. doi: 10.1128/spectrum.03055-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kareinen L, Tammiranta N, Kauppinen A, Zecchin B, Pastori A, Monne I, Terregino C, Giussani E, Kaarto R, Karkamo V, et al. 2024. Highly pathogenic avian influenza A(H5N1) virus infections on fur farms connected to mass mortalities of black-headed gulls, Finland, July to October 2023. Euro Surveill 29:2400063. doi: 10.2807/1560-7917.ES.2024.29.25.2400063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shah S, Gwee SXW, Ng JQX, Lau N, Koh J, Pang J. 2022. Wastewater surveillance to infer COVID-19 transmission: a systematic review. Sci Total Environ 804:150060. doi: 10.1016/j.scitotenv.2021.150060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Louis S, Mark-Carew M, Biggerstaff M, Yoder J, Boehm AB, Wolfe MK, Flood M, Peters S, Stobierski MG, Coyle J, et al. 2024. Wastewater surveillance for influenza A virus and H5 subtype concurrent with the highly pathogenic avian influenza A(H5N1) virus outbreak in cattle and poultry and associated human cases - United States, May 12-July 13, 2024. MMWR Morb Mortal Wkly Rep 73:804–809. doi: 10.15585/mmwr.mm7337a1 [DOI] [PMC free article] [PubMed] [Google Scholar]