Abstract
Background
Antihyperglycemic agents with cardiovascular (CV) benefits, including SGLT-2i and GLP-1RA, are underused in clinical practice, particularly by cardiologists. Understanding the prescribing patterns of these agents by cardiologists may aid in implementation efforts.
Methods
The COORDINATE-Diabetes trial enrolled participants with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) from US cardiology clinics and evaluated the impact of cluster randomization to a multifaceted implementation intervention versus usual care on proportional prescription of evidence-based therapies; the present analyses focus on SGLT2i and GLP-1 RA prescription. Participants prescribed an SGLT-2i were pooled between study arms and compared with those not initiating. A logistic regression model with random intercepts for the site was fitted with adjustment for treatment assignment (intervention vs. usual care), demographics, medical history, baseline medications and site location to determine factors associated with starting SGLT-2i. The same analysis was performed to determine factors associated with prescribing GLP-1RA. Reasons for not commencing either SGLT-2i or GLP-1RA in the intervention arm were aggregated and reported as counts.
Results
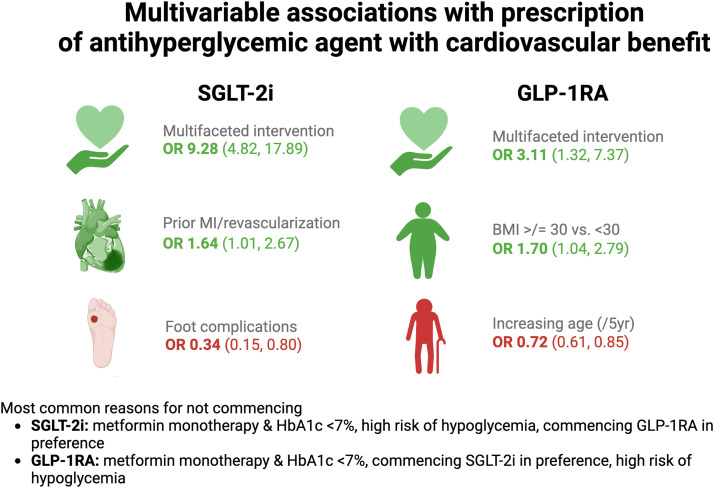
Of all 1045 participants enrolled between July 2019 and May 2022 and followed for 6–12 months, 290 (27.8 %) were prescribed an SGLT-2i and 118 (11.3 %) a GLP-1RA; 8 (0.8 %) were prescribed both. Enrollment at an intervention site was an important predictor of SGLT-2i (OR 9.28, 4.82–17.89) and GLP-1RA prescription (OR 3.11, 1.32–7.37). Prior MI/coronary revascularization (OR 1.64, 1.01–2.67) was significantly associated with SGLT-2i prescription. A trend towards significance was observed for the association of preserved kidney function (OR 1.47, 0.99–2.19) and higher Charlson comorbidity index (OR 1.54, 0.97–2.44) with higher odds of SGLT-2i prescription. In contrast, T2D foot complications (OR 0.34, 0.15–0.80) were significantly associated with lower odds of SGLT-2i prescription. Older age was also directionally associated (OR 0.91, 0.81–1.02) with lower prescription of SGLT-2i. With respect to GLP-1RA, the presence of obesity (OR 1.70, 1.04–2.79) was associated with prescription, while increasing age (OR 0.72, 0.61–0.85) was associated with lower odds of prescription. The most common identifiable reason for not prescribing either SGLT-2i or GLP-1RA was related to now outdated guidance (i.e. permissible exclusion if metformin monotherapy and HbA1c <7 %). Contraindications to either agent and high cost were infrequently cited as reasons for not prescribing.
Conclusion
Consistent with the main trial results, participation in the COORDINATE-Diabetes intervention arm was an important determinant of higher odds for SGLT-2i and GLP-1RA prescription. Patient-level characteristics appeared to modestly influence the likelihood of prescription and may benefit from targeted education content.
Keywords: Type 2 diabetes mellitus, Sodium glucose co-transporter 2 inhibitor, Glucagon-like peptide-1 receptor agonist, Prescription, Implementation
Central Illustration. Multivariable associations with prescription of antihyperglycemic agent with CV benefit (SGLT-2i or GLP-1RA). Presented as odds ratio [OR] and (95 % confidence interval).
1. Introduction
An established evidence base supports the use of sodium glucose co-transporter-2 inhibitors (SGLT-2i)[1] and glucagon-like peptide-1 receptor agonists (GLP-1RA)[2] to reduce cardiovascular and kidney disease risk among persons with type 2 diabetes (T2D) and (or at high risk of) atherosclerotic cardiovascular disease (ASCVD). Yet despite their clear efficacy and favorable safety profiles, these agents are underused in clinical practice[3,4], and specifically by cardiologists[5]. Given their important role in the care of persons with T2D with or at high risk for ASCVD, cardiologists are well-positioned to commence SGLT-2i or GLP-1RA. However, a lack of familiarity with these agents and concern of overstepping disciplinary boundaries contributes to suboptimal implementation[6].
To address these adoption barriers, the Coordinating Cardiology Clinics Randomized Trial of Interventions to Improve Outcomes (COORDINATE)–Diabetes was designed, a cluster randomized clinical trial of a multifaceted intervention to increase use of evidence based CV therapies compared with usual care among persons with T2D and ASCVD conducted at cardiology clinics in the US. At follow-up, participants in the intervention group were significantly more likely to be prescribed evidence-based therapies, including SGLT-2i and/or GLP-1RA, compared with those in the control group, 37.9 vs. 14.5 %[7]. Here, we report the characteristics of participants newly prescribed an SGLT-2i and/or GLP-1RA during study follow-up and the reasons underlying the lack of their initiation. Understanding the prescribing decisions of cardiologists may help refine future intervention elements to achieve broad, equitable, and consistent uptake of SGLT-2i and GLP-1RA.
2. Methods
2.1. Study design and participants
These were prespecified analyses from the COORDINATE Diabetes clinical trial. The trial design[7] and primary results of the main trial have been published previously[4]. As mentioned, COORDINATE Diabetes was a cluster randomized clinical trial with 43 cardiology clinics in the United States that were randomly assigned to intervention or usual care (1:1). The multifaceted intervention included a suite of strategies aimed to a) assess local barriers to the uptake of evidence-based therapies, b) develop local interdisciplinary care pathways, c) coordinate care among clinicians, d) educate clinicians on guidelines and practice updates, e) report data back to clinics, and f) provide tools for participants. Adult participants with T2D and ASCVD were eligible for enrolment from July 2019 until May 2022, and follow up was completed December 2022. The primary outcome of the trial was the proportion of patients on all three evidence-based therapies: high-intensity statin, angiotensin converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB) and either SGLT-2i or GLP-1RA (or metformin monotherapy with HbA1c <7 %). Because we systematically collected reasons for not starting these treatments, this study provides a unique opportunity to understand patterns of use.
2.2. Outcomes
The two primary outcomes of this study were a prescription at any time during follow up as documented in the electronic health record for a) SGLT-2i and b) GLP-1RA. Secondary outcomes were the reasons for not commencing SGLT-2i or GLP-1RA among participants in the intervention arm. At the end of each study visit (3, 6, 9 and 12 months), intervention sites were asked to document the reason(s) why the participant was not commenced on an evidence-based therapy on the case report form. Pre-populated options (tick all that apply) were provided in the case report form, along with a free text for ‘other’. Free text reasons were subsequently evaluated and grouped by themes. Metformin monotherapy with HbA1c <7% was considered an acceptable reason for not commencing SGLT-2i or GLP-1RA at the commencement of the trial in 2019; however, as the trial progressed, sites were encouraged to prescribe therapies for these patients consistent with the evolving guidelines.
2.3. Variables
Coronary artery disease (CAD) was defined as prior MI, coronary revascularization (CABG or PCI), and/or obstructive disease (≥50 %) as documented by invasive or CT angiography; cerebrovascular disease (CeVD) was defined as stroke and/or carotid artery stenosis (≥50 %); and peripheral artery disease (PAD) was defined as claudication with ankle-brachial index <0.9, prior peripheral revascularization, and/or amputation due to circulatory insufficiency. Race and ethnicity were self-reported. Urban vs. suburban/rural clinic location was self-reported by the sites. Charlson comorbidity index (CCI) was calculated as previously described[8] and is the sum of points assigned by age and a number of diseases including connective tissue disorders, dementia, peptic ulcer, liver disease, hemiplegia, chronic kidney disease (CKD), tumor and HIV among others. A value of 5 or more is considered an index of severe comorbidity. Foot complications were defined as a history of ulcers, calluses or amputation.
2.4. Statistical analysis
All participants commencing an SGLT-2i were identified and compared with those not commencing an SGLT-2i in follow-up. Baseline comparisons between groups were summarized as mean (SD), median (Q1,Q3) for continuous variables, and counts (percentages) for categorical variables. Differences in characteristics between study groups were assessed using the Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables. The same process was repeated to identify participants commencing GLP-1RA who were then compared with those not commencing GLP-1RA.
To determine the factors associated with prescribing an SGLT-2i, a logistic regression model was fit in the overall population with random intercepts for the site. Adjustment for the following clinically relevant variables was performed; trial allocation (intervention or usual care), demographic characteristics (age, sex, race, Hispanic or Latino ethnicity, health insurance, medical history (Charlson comorbidity index ≥5, atherosclerotic cardiovascular disease phenotype [(CAD vs. CeVD vs. PAD)], atrial fibrillation, heart failure, T2D foot complications, body mass index (BMI) [≥30 vs <30], CKD [eGFR ≥60 vs. <60]) and medications (statin, ACEi/ARB, metformin monotherapy, insulin, ≥2 oral antihyperglycemics, and site characteristics (urban vs. non-urban/rural). Model output is reported as odds ratio (95 %CI) with F-value test statistics. The same analysis was repeated to determine the factors associated with prescribing a GLP-1RA. Interaction testing evaluated if the associations of characteristics and prescribing patterns differed in the intervention versus control patients. A sensitivity analysis excluding individuals on metformin monotherapy with an HbA1c <7% was performed as contemporary guidance did not recommend SGLT-2i or GLP-1RA in that context.
Participants in the intervention arm who were not prescribed SGLT-2i or GLP-RA were identified. Reasons for not commencing either SGLT-2i or GLP-1RA during follow-up were aggregated throughout the trial and reported as counts. Multiple reasons for not commencing SGLT-2i or GLP-1RA could be listed for a participant.
3. Results
3.1. Patterns of SGLT-2i prescription
A total of 290/1045 (27.8 %) participants were prescribed an SGLT-2i during follow up; 218 (75.2 %) were in the intervention arm, while 72 (24.8 %) were in the usual care arm. The characteristics of participants prescribed an SGLT-2i during the study follow-up are presented in Table 1. Participants with (vs. without) SGLT-2i prescription during the study period were slightly younger, more commonly male and of Hispanic/Latino ethnicity, and had a higher prevalence of heart failure. Among participants prescribed SGLT-2i, Medicare insurance was less common, while no insurance was more common. Chronic kidney disease (eGFR <60), T2D foot complications, and neuropathy were less common among those prescribed an SGLT-2i. HbA1c was higher in the SGLT-2i group; however, the composition of the antihyperglycemic regimen overall was comparable at baseline, including the proportion on insulin.
Table 1.
Characteristics of participants who were ever prescribed an SGLT-2i compared with those who were never prescribed an SGLT-2i.
| Overall (N = 1045) |
Ever prescribed SGLT-2i (N = 290) | Never prescribed SGLT-2i (N = 755) | P value | |
|---|---|---|---|---|
| Intervention arm | 457 (43.7) | 218 (75.2) | 239 (31.7) | <0.001 |
| Age – yr, mean±SD | 69.4 ± 9.5 | 68.4 ± 9.5 | 69.7 ± 9.5 | 0.031 |
| Female sex – no. ( %) | 338 (32.3) | 79 (27.2) | 259 (34.3) | 0.029 |
| Race* – no. ( %) | ||||
| Asian | 27 (2.6) | 10 (3.4) | 17 (2.3) | 0.275 |
| American Indian/Alaskan native | 7 (0.7) | 1 (0.3) | 6 (0.8) | 0.425 |
| Black | 172 (16.5) | 238 (82.1) | 635 (84.1) | 0.427 |
| White | 801 (76.4) | 207 (71.4) | 594 (78.7) | 0.013 |
| Hispanic or Latino* – no. ( %) | 90 (8.6) | 32 (11.0) | 58 (7.7) | <0.001 |
| Urban vs. non-urban – no. ( %) | 555 (53.1) | 143 (49.3) | 347 (46.0) | 0.331 |
| Health Insurance – no. ( %) | ||||
| Medicare | 689 (67.4) | 170 (61.2) | 519 (69.8) | 0.009 |
| Private | 349 (34.1) | 103 (37.1) | 246 (33.1) | 0.232 |
| Medicaid | 107 (10.5) | 32 (11.5) | 75 (10.1) | 0.507 |
| None | 23 (2.2) | 12 (4.1) | 11 (1.5) | 0.008 |
| Atherosclerotic disease – no. ( %) | ||||
| Coronary | 927 (88.7) | 263 (90.7) | 664 (87.9) | 0.210 |
| Prior MI/revascularization | 865 (82.8) | 242 (83.4) | 623 (82.5) | 0.721 |
| Cerebrovascular | 273 (26.1) | 72 (24.8) | 201 (26.6) | 0.554 |
| Peripheral | 85 (8.1) | 26 (9.0) | 59 (7.8) | 0.542 |
| Atrial fibrillation – no. ( %) | 219 (21.0) | 58 (20.0) | 161 (21.3) | 0.638 |
| Heart failure – no. ( %) | 294 (28.1) | 99 (34.1) | 195 (25.8) | 0.008 |
| Hypertension – no. ( %) | 978 (93.6) | 274 (94.5) | 704 (93.2) | 0.465 |
| Hyperlipidemia – no. ( %) | 955 (91.0) | 264 (91.0) | 688 (91.1) | 0.963 |
| Charlson comorbidity modified index – no. ( %) | ||||
| Severe, CCI≥5 | 625 (59.8) | 170 (58.6) | 455 (60.3) | 0.628 |
| Diabetes history – no. ( %) | ||||
| DKA | 9 (0.9) | 2 (0.7) | 7 (0.9) | 0.710 |
| Retinopathy | 59 (5.6) | 16 (5.5) | 43 (5.7) | 0.911 |
| Neuropathy | 270 (25.8) | 50 (17.2) | 220 (29.1) | <0.001 |
| Foot complications | 62 (5.9) | 9 (3.1) | 53 (7.0) | 0.017 |
| Gastroparesis | 22 (2.1) | 5 (1.7) | 17 (2.3) | 0.595 |
| Anthropometric and labs | ||||
| HbA1c Mean %±SD [850] | 7.6 ± 1.4 | 7.9 ± 1.6 | 7.5 ± 1.4 | <0.001 |
| eGFR Mean mL/min/1.73m2 ±SD [905] | 66.1 ± 19.2 | 67.6 ± 17.7 | 65.5 ± 19.7 | 0.092 |
| <60 | 313 (30.0) | 72 (24.8) | 241 (31.9) | 0.016 |
| BMI mean kg/m2±SD | 32.3 ± 6.9 | 32.1 ± 6.5 | 32.4 ± 7.1 | 0.775 |
| ≥30 | 592 (56.7) | 156 (53.8) | 436 (57.7) | 0.332 |
| Baseline medications. ( %) | ||||
| Statin | 968 (92.6) | 273 (94.1) | 695 (92.1) | 0.248 |
| Non-statin LLT | 223 (21.3) | 51 (17.6) | 172 (22.8) | 0.067 |
| Aspirin | 768 (86.8) | 221 (87.7) | 547 (86.4) | 0.611 |
| ACEi/ARB | 752 (72.0) | 214 (73.8) | 500 (66.2) | 0.414 |
| Antihyperglycemic | ||||
| Metformin | 623 (59.6) | 168 (57.9) | 455 (60.3) | 0.491 |
| Sulfonylurea | 313 (30.0) | 93 (32.1) | 220 (29.1) | 0.355 |
| Thiazolidinedione | 28 (2.7) | 6 (2.1) | 22 (2.9) | 0.449 |
| DPP-4i | 125 (12.0) | 27 (9.3) | 98 (13.0) | 0.101 |
| Insulin | 371 (35.5) | 101 (34.8) | 270 (35.8) | 0.778 |
| ≥ 2 oral hyperglycemics | 263 (25.2) | 66 (22.8) | 197 (26.1) | 0.266 |
ACEi/ARB – angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI – body mass index; CCI- charlson comorbidity index; DPP4i – dipeptidyl peptidase 4 inhibitor; eGFR – estimated glomerular filtration rate; LLT – lipid lowering therapy; MI – myocardial infarction.
Treatment at an intervention site accounted for most of the variance in the multivariable model (Fig. 1) and was associated with the highest odds of SGLT-2i prescription in follow-up (OR 9.28, 4.82–17.89). History of prior MI/revascularization (OR 1.61, 1.01–2.67) was statistically significantly associated with higher odds of SGLT-2i prescription while trends for higher use were observed for male sex (OR 1.41, 0.98–2.04), preserved eGFR ≥60 (OR 1.47, 0.99–2.19) and higher comorbidity index (OR 1.54, 0.97–2.44). In contrast, the presence of T2D foot complications was significantly associated with lower odds of SGLT-2i prescription (OR 0.34, 0.15–0.80) while there was a trend towards lower odds of prescription with older age (OR 0.91, 0.81–1.02). Results from a sensitivity analysis excluding those on metformin monotherapy with HbA1c <7 % (N = 837) were largely similar, except that metformin use at baseline became a significant predictor of SGLT-2i prescription (See Supplement).
Fig. 1.
Multivariable associations with ever prescription of SGLT-2i in follow up.
3.2. Patterns of GLP-1RA prescription
A total of 118/1045 (11.3 %) participants were prescribed a GLP-1RA during follow-up; 85 (72.0 %) were in the intervention arm, while 33 (28.0 %) were in the usual care arm. Characteristics of participants who ever (compared with never) prescribed a GLP-1RA during study follow-up are presented in Table 2. During the study period, participants with (vs. without) GLP-1RA prescription were significantly younger and more often Hispanic or Latino but less frequently White or with Medicare insurance. Rates of multi-morbidity were lower among those prescribed GLP-1RA, as were rates of hypertension. The overall BMI was higher in the GLP-1RA group, as was the proportion of individuals with Class I (or higher) obesity. GLP-1RA users had higher HbA1c and eGFR at baseline than those never prescribed the class.
Table 2.
Characteristics of participants ever prescribed a GLP-1RA compared with those never prescribed a GLP-1RA.
| Overall (N = 1045) |
Ever prescribed GLP-1RA (N = 118) | Never prescribed GLP-1RA (N = 927) | P value | |
|---|---|---|---|---|
| Intervention arm | 457 (43.7) | 85 (72.0) | 372 (40.1) | |
| Age – yr, mean±SD | 69.4 ± 9.5 | 65.4 ± 9.8 | 69.9 ± 9.3 | <0.001 |
| Female sex – no. ( %) | 338 (32.3) | 44 (37.3) | 294 (31.7) | 0.223 |
| Race* – no. ( %) | ||||
| Asian | 27 (2.6) | 5 (4.2) | 22 (2.4) | 0.230 |
| American Indian/Alaskan native | 7 (0.7) | 0 (0) | 7 (0.8) | 0.344 |
| Black | 172 (16.5) | 20 (16.9) | 152 (16.4) | 0.879 |
| White | 801 (76.4) | 80 (67.8) | 721 (77.8) | 0.016 |
| Hispanic or Latino* – no. ( %) | 90 (8.6) | 16 (13.6) | 74 (8.0) | 0.001 |
| Urban (vs. non-urban) – no. ( %) | 490 (46.9) | 44 (37.3) | 446 (48.1) | 0.027 |
| Health Insurance – no. ( %) | ||||
| Medicare | 689 (67.4) | 66 (56.4) | 623 (68.8) | 0.007 |
| Private | 349 (34.1) | 48 (41.0) | 301 (33.3) | 0.096 |
| Medicaid | 107 (10.5) | 10 (8.5) | 97 (10.7) | 0.356 |
| None | 23 (2.2) | 1 (0.8) | 22 (2.4) | 0.288 |
| Atherosclerotic disease – no. ( %) | ||||
| Coronary | 927 (88.7) | 103 (87.3) | 824 (88.9) | 0.605 |
| Prior MI/revascularization | 865 (82.8) | 97 (82.2) | 768 (82.8) | 0.861 |
| Cerebrovascular | 273 (26.1) | 28 (23.7) | 245 (26.4) | 0.530 |
| Peripheral | 139 (13.3) | 18 (15.3) | 121 (13.1) | 0.507 |
| Atrial fibrillation – no. ( %) | 219 (21.0) | 21 (17.8) | 198 (21.4) | 0.371 |
| Heart failure – no. ( %) | 294 (28.1) | 29 (24.6) | 265 (28.6) | 0.362 |
| Hypertension – no. ( %) | 978 (93.6) | 105 (89.0) | 873 (94.2) | 0.030 |
| Hyperlipidemia – no. ( %) | 955 (91.0) | 105 (89.0) | 847 (91.4) | 0.391 |
| Charlson comorbidity modified index – no. ( %) | ||||
| Severe, CCI≥5 | 625 (59.8) | 56 (47.5) | 569 (61.4) | 0.004 |
| Diabetes history – no. ( %) | ||||
| DKA | 9 (0.9) | 0 (0) | 9 (1.0) | 0.283 |
| Retinopathy | 59 (5.6) | 7 (5.9) | 52 (5.6) | 0.886 |
| Neuropathy | 270 (25.8) | 31 (26.3) | 239 (25.8) | 0.909 |
| Foot complications | 62 (5.9) | 5 (4.2) | 57 (6.1) | 0.408 |
| Gastroparesis | 22 (2.1) | 0 (0) | 22 (2.4) | 0.091 |
| Anthropometric and labs | ||||
| HbA1c Mean %±SD [850] | 7.6 ± 1.4 | 8.3 ± 1.6 | 7.5 ± 1.4 | <0.001 |
| eGFR Mean mL/min/1.73m2 ±SD [905] | 66.1 ± 19.2 | 69.8 ± 20.6 | 65.6 ± 19.0 | 0.048 |
| <60 | 313 (30.0) | 30 (25.4) | 283 (30.5) | 0.244 |
| BMI mean kg/m2±SD | 32.3 ± 6.9 | 34.4 ± 8.5 | 32.1 ± 6.7 | 0.008 |
| ≥30 | 592 (56.7) | 78 (66.1) | 541 (55.4) | 0.026 |
| Baseline medications. ( %) | ||||
| Statin | 968 (92.6) | 111 (94.1) | 857 (92.4) | 0.526 |
| Non-statin LLT | 223 (21.3) | 29 (24.6) | 194 (20.9) | 0.363 |
| Aspirin | 768 (86.8) | 92 (90.2) | 676 (86.3) | 0.279 |
| ACEi/ARB | 752 (72.0) | 94 (79.7) | 658 (71.0) | 0.048 |
| Antihyperglycemic | ||||
| Metformin | 623 (59.6) | 72 (61.0) | 551 (59.4) | 0.742 |
| Sulfonylurea | 313 (30.0) | 37 (31.4) | 276 (29.8) | 0.724 |
| Thiazolidinedione | 28 (2.7) | 3 (3.3) | 25 (2.7) | 0.922 |
| DPP-4i | 125 (12.0) | 10 (8.5) | 115 (12.4) | 0.215 |
| Insulin | 371 (35.5) | 49 (41.5) | 322 (34.7) | 0.145 |
| ≥ 2 oral hyperglycemics | 263 (25.2) | 27 (22.9) | 236 (25.5) | 0.544 |
ACEi/ARB – angiotensin-convering enzyme inhibitor/angiotensin receptor blocker; BMI – body mass index; CCI- charlson comorbidity index; DPP4i – dipeptidyl peptidase 4 inhibitor; eGFR – estimated glomerular filtration rate; LLT – lipid lowering therapy; MI – myocardial infarction.
In the multivariable model (Fig. 2), treatment at an intervention site had the highest odds of GLP-1RA prescription in follow-up (OR 3.11, 1.32–7.37). The only other factor associated with significantly higher odds of GLP-1RA prescription was a BMI ≥30 (OR 1.70, 1.04–2.79)). Most of the model variance was determined by age, which was strongly and inversely associated with the odds of GLP-1RA prescription (OR 0.72, 0.61–0.85). Sensitivity analysis excluding those on metformin monotherapy with HbA1c <7 % (N = 806) did not significantly impact the model parameters (See Supplement).
Fig. 2.
Multivariable associations with ever prescription of GLP-1RA in follow up.
3.3. Reasons for not prescribing SGLT-2i or GLP-1RA
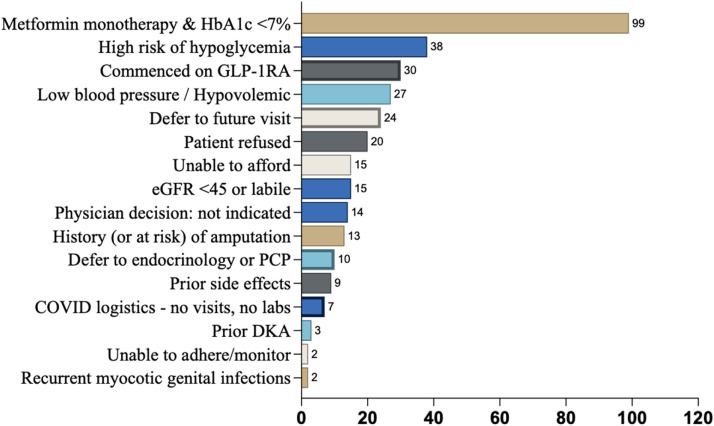
A total of 239 (52.3%) participants in the intervention group were never prescribed an SGLT-2i in follow-up. The most prevalent reason provided for not commencing SGLT-2i among these participants was ‘Metformin monotherapy with an HbA1c <7%’ (n = 99) (Fig. 3). Other mentioned reasons for not prescribing SGLT-2i included clinical scenarios listed in the product labeling information in which precaution should be exercised, such as ‘high risk of hypoglycemia’ (n = 38), ‘low blood pressure/hypovolemia’ (n = 27) and ‘high risk of amputation’ (n = 13). The absolute contraindication (at the time of COORDINATE Trial) of eGFR <45ml/min/m2 (n = 15) was infrequently cited, as was the relative contraindication of ‘prior DKA’ (n = 3). Inability to afford was listed for 15 participants, while 20 chose not to commence an SGLT-2i. Deferral to a future visit (n = 24) or to another clinician (n = 10) was common. The commencement of a GLP-1RA was listed as the reason for not prescribing SGLT-2i in 30 participants.
Fig. 3.
Reasons for not commencing SGLT-2i at each visit (intervention arm only).
A total of 372 (81.4 %) participants in the intervention group were never prescribed a GLP-1RA in follow-up. As above, the most common reason for not commencing GLP-1RA was ‘Metformin monotherapy with an HbA1c <7 %’ (n = 187), Fig. 4. Clinical scenarios listed in the product labeling information in which precaution should be exercised included ‘high risk of hypoglycemia’ (n = 61), ‘eGFR <30’ (n = 8), gastroparesis/gastric surgery (n = 7), and history of pancreatitis (n = 1). Deferral to a future visit (n = 30) or to another clinician (n = 11) was common, as was the commencement of an SGLT-2i as an explanation for not prescribing GLP-1RA (n = 75). 29 participants could not afford a GLP-1RA, 9 reported prior side effects, and 41 decided against GLP-1RA for reasons not further described by the site.
Fig. 4.
Reasons for not commencing GLP-1RA at each visit (intervention arm only).
4. Discussion
This study describes the characteristics of participants prescribed or not prescribed either SGLT-2i and GLP-1RA during follow-up of the COORDINATE-Diabetes implementation trial. We report the following key findings: 1) reasons for not commencing SGLT-2i and GLP-1RA reveal few absolute contraindications and multiple opportunities to improve uptake further; 2) the presence of T2D foot complications appeared to be a strong deterrent to SGLT-2i prescription; 3) older participants were less likely to receive GLP-1RA with a similar trend observed for SGLT-2i; 4) females appeared less likely to be prescribed SGLT-2i compared with males; and 5) guideline-recommended phenotypes for choice of SGLT-2i vs. GLP-1RA were evident in prescribing behavior with some exceptions.
The most common reason for not commencing either SGLT-2i or GLP-1RA was treatment with metformin monotherapy and an HbA1c <7 %. While contemporary guidance recommends the addition of either agent independent of HbA1c or baseline pharmacotherapy, contemporary guidance when the COORDINATE-Diabetes was designed suggested individuals should be on metformin first. Further evidence of this behavior is in the sensitivity analysis which revealed baseline metformin use to be associated with prescription of SGLT-2i in follow up. It is also probable that cardiologists were less comfortable initiating an SGLT-2i without metformin already on board (or even less comfortable initiating both). While this was not observed with GLP-1RA, the frequency of GLP-1RA prescription was considerably lower. Similarly, very few patients were on both SGLT2i and GLP-1RA, an approach now recommended in the guidelines, with the commencement of one agent commonly listed as a reason for not prescribing the other. Thus, given labelling precautions and absolute contraindications were comparably infrequent, iteration of our intervention to focus on contemporary guidance could be expected to overcome the majority of reasons for non-commencement of either agent. Clinical inertia was evident with multiple opportunities for commencing therapies devolved to other clinicians or to future visits. Notably, while a non-trivial number of patients were reportedly unable to afford SGLT-2i or GLP-1RA, this was not the dominant cause for a lack of prescription. Intervention sites were educated on ways to increase access (e.g. discount cards, insurance appeals) and thus this number may be lower than the general population, however our data should caution an overemphasis on affordability as the sole cause of the slow uptake of these agents.
A history of T2D foot complications was associated with over 60% lower odds of receiving a prescription for SGLT-2i. This prescribing behavior is in-line with persistent concern generated from the CANVAS program in which canagliflozin-treated patients had a higher rate of lower limb amputation compared with control[9] and from which a regulatory prescribing warning was issued. Subsequent post-marketing experience has not confirmed an amputation risk for the SGLT-2i class[10,11] although a definitive understanding of the CANVAS signal remains elusive. Of note in the CREDENCE trial, that also evaluated canagliflozin, there was no signal for amputation[12] although a protocol amendment required physicians to examine the lower limbs at each visit and pause investigational products during foot complications. Thus, it’s unclear whether the amputation risk observed in CANVAS was mitigated by the close surveillance employed in the CREDENCE protocol or simply the play of chance in the CANVAS study. Nonetheless, this signal was not observed with other agents in the class, and thus, the FDA warning was removed in 2020. While hesitancy to prescribe SGLT-2i to patients with active T2D foot complications is reasonable and reflected the contemporary evidence, an important related finding was that this hesitancy did not unnecessarily extend to patients with stable PAD who appeared similarly likely to receive an SGLT-2i and are likely to benefit most from cardiovascular and kidney disease protection.
Older patients with T2D are at an increased risk of cardiovascular and kidney complications and thus represent a group more likely to benefit from therapies that reduce this risk[13]. Despite this elevated risk, older patients in our study were almost 30% less likely to receive a GLP-1RA and up to 10% less likely to receive an SGLT-2i. This is consistent with results from observational analyses that show lower use of SGLT-2i or GLP-1RA among older patients (>65 years) in clinical practice[14,15]. Reasons for less likely use may relate to a need for more confidence in the data among this age group owing to the under-representation of older patients in the seminal trials of SGLT-2i and GLP-1RA. However, in pooled analyses of trial data that have included up to 1/3 of participants aged 65 years or older[16,17], no evidence of interaction by age suggests consistent relative benefit (and greater absolute benefit given greater baseline risk) among older patients. Furthermore, while adverse events are more frequent in older patients (e.g. volume depletion, DKA, hypoglycemia), they appear no higher in active treatment arms than in placebo[[18], [19], [20]]. Frailty may identify a subset of older patients at higher risk of complications and for whom lower use has been previously documented[21]. While not explicitly captured in the COORDINATE trial, the presence of severe multi-morbidity as a surrogate for frailty did not negatively impact prescription. Thus, in the context of an aging population and the rising prevalence of diabetes, the presence of a risk-treatment paradox among older patients represents a significant opportunity for meaningful cardiorenal risk reduction.
Women appeared less likely to be prescribed an SGLT-2i in follow up compared with men. This finding is consistent with our own earlier data[3,4] and that of others in the US and Europe, which demonstrate lower use of SGLT-2i among women with diabetes[22,23] and with heart failure[24]. While these studies have generally evaluated ‘use’, a combination of both clinician prescription and patient adherence, our finding of lower odds of prescription relates to clinician-only behavior; it may reflect a lower clinician-perceived risk of cardiovascular disease or heightened concern for adverse events among women[25]. Importantly, results from pooled analyses of SGLT-2i trials demonstrate consistent cardiovascular and kidney disease risk reduction and a similar side effect profile[14] by sex providing no scientific rationale for differential prescription rates. Further work must be directed to addressing sex-based differences in evidence-based therapy adoption.
Cardiovascular guidelines recommend phenotyping patients with diabetes to determine preference for either SGLT-2i or GLP-1RA for cardiovascular benefit where administration of both is not feasible[6,26]. In that context, those with (or at risk for) heart failure or chronic kidney disease may be better served with an SGLT-2i while those in whom ASCVD or obesity-driven risk predominates, a GLP-1RA may be preferable. Consistent with this hypothesis, we observed those with a BMI ≥30 were 70 % more likely to receive a GLP-1RA prescription compared with those noted to have a BMI <30 in our study. Similarly, heart failure was more prevalent among those prescribed an SGLT-2i with a strong trend towards an independent association in the multivariable model. In contrast however, eGFR <60 was associated with a lower likelihood of SGLT-2i prescription despite this group being more likely to benefit from nephroprotection in contemporary guidelines. These practice patterns may reflect the evolving evidence base that was being generated during the trial as heart failure and kidney-specific data for these agents were progressively reported, but nonetheless highlights an opportunity to improve use in a population who may derive particular benefit.
The results of these analyses should be interpreted in the context of several limitations. Prescribing behavior, even those in the usual care arm, were observed in the context of a clinical trial and are subject to Hawthorn effect. Similarly, the clinicians who signed up to participate in the COORDINATE-Diabetes trial are likely to be more engaged and literate regarding the use of SGLT-2i and GLP-1RA and thus prescription rates observed are likely higher than in routine clinical practice. These analyses specifically looked at prescribing and did not evaluate adherence of these agents. Thus, our estimate of ‘use’ is likely to be an over- rather than underestimate. Randomization occurred at a site level rather than a patient level thus a degree of residual confounding between aspects of the intervention and prescription is likely to exist despite multivariable modelling and adjustment. Notwithstanding these limitations, the strength of our analysis is the granular and actionable insights into cardiologists’ prescribing practices that aren’t discernible from observational datasets.
In summary, participation in the COORDINATE-Diabetes intervention arm was a large determinant of GLP-1RA and SGLT-2i prescription. While there was evidence of prescribing behavior that reflected guideline recommended ‘phenotypes’ at the time, lesser use of SGLT-2i particularly among women and older participants is important to address with education. Contraindications to SGLT-2i and GLP-1RA were rare while clinical inertia was common; thus, most individuals could likely be commenced on either agent.
Role of funder
Funder participated in the design and conduct of the study; interpretation of the data; and review or approval of the manuscript. The funder had no role in the collection, management, analysis; preparation of the manuscript; and decision to submit hte manuscript for publication.
CRediT authorship contribution statement
Adam J Nelson: Writing – original draft, Investigation, Visualization, Data curation, Methodology. Lisa A. Kaltenbach: Formal analysis. Hussein R. Al-Khalidi: Formal analysis, Conceptualization. Monica Leyva: Project administration, Writing – review & editing, Methodology. Laura Webb: Project administration. Darren K. McGuire: Investigation, Writing – review & editing. Rodica Pop-Busui: Investigation, Writing – review & editing. Matthew A Cavender: Writing – review & editing. Vanita R. Aroda: Writing – review & editing. Melissa L. Magwire: Investigation. Caroline R. Richardson: Writing – review & editing. Ildiko Lingvay: Writing – review & editing, Investigation. Julienne K. Kirk: Writing – review & editing. Ambarish Pandey: Writing – review & editing. Tanya Gaynor: Writing – review & editing. Jonathan Pak: Writing – review & editing. Alana Washington: Writing – review & editing. Cagri Senyucel: Writing – review & editing. Renato D. Lopes: Writing – review & editing. Jennifer B. Green: Writing – review & editing, Data curation, Investigation. Christopher B. Granger: Methodology, Data curation, Writing – review & editing, Funding acquisition, Conceptualization. Neha J Pagidipati: Writing – review & editing, Investigation, Conceptualization, Methodology, Supervision, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
The COORDINATE-Diabetes trial is an external collaborative research study by the Duke Clinical Research Institute and sponsored by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and Eli Lilly. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all aspects of the trial and publication development.
Acknowledgements
The COORDINATE-Diabetes Steering Committee would like to thank all participating clinics, investigators, clinicians, nurses, trial coordinators, and the participants who made the trial possible.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2025.101058.
Appendix. Supplementary materials
References
- 1.McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattar N., Lee M.M.Y., Kristensen S.L., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 3.Nelson A.J., Ardissino M., Haynes K., et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.016835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson A.J., O'Brien E.C., Kaltenbach L.A., et al. Use of lipid-, blood pressure-, and glucose-lowering pharmacotherapy in patients with type 2 diabetes and atherosclerotic cardiovascular disease. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.48030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari R., Jha K., Dardari Z., et al. National trends in use of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists by cardiologists and other specialties, 2015 to 2020. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.023811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson A.J., Pagidipati N.J., Aroda V.R., et al. Incorporating SGLT2i and GLP-1RA for cardiovascular and kidney disease risk reduction: call for action to the cardiology community. Circulation. 2021;144:74–84. doi: 10.1161/CIRCULATIONAHA.121.053766. [DOI] [PubMed] [Google Scholar]
- 7.Nelson A.J., Pagidipati N.J., Kelsey M.D., et al. Coordinating cardiology clinics randomized trial of interventions to improve outcomes (COORDINATE) - diabetes: rationale and design. Am Heart J. 2023;256:2–12. doi: 10.1016/j.ahj.2022.10.079. [DOI] [PubMed] [Google Scholar]
- 8.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 10.Paul S.K., Bhatt D.L., Montvida O. The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur Heart J. 2021;42:1728–1738. doi: 10.1093/eurheartj/ehaa956. [DOI] [PubMed] [Google Scholar]
- 11.Fadini G.P., Avogaro A. SGLT2 inhibitors and amputations in the US FDA adverse event reporting system. Lancet Diabetes Endocrinol. 2017;5:680–681. doi: 10.1016/S2213-8587(17)30257-7. [DOI] [PubMed] [Google Scholar]
- 12.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 13.Scheen A.J., Bonnet F. Efficacy and safety profile of SGLT2 inhibitors in the elderly: how is the benefit/risk balance? Diabetes Metab. 2023;49 doi: 10.1016/j.diabet.2023.101419. [DOI] [PubMed] [Google Scholar]
- 14.Radholm K., Zhou Z., Clemens K., Neal B., Woodward M. Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes Obes Metab. 2020;22:263–266. doi: 10.1111/dom.13876. [DOI] [PubMed] [Google Scholar]
- 15.Limonte C.P., Hall Y.N., Trikudanathan S., et al. Prevalence of SGLT2i and GLP1RA use among US adults with type 2 diabetes. J Diabetes Complicat. 2022;36 doi: 10.1016/j.jdiacomp.2022.108204. [DOI] [PubMed] [Google Scholar]
- 16.Karagiannis T., Tsapas A., Athanasiadou E., et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pr. 2021;174 doi: 10.1016/j.diabres.2021.108737. [DOI] [PubMed] [Google Scholar]
- 17.Giugliano D., Longo M., Maiorino M.I., et al. Efficacy of SGLT-2 inhibitors in older adults with diabetes: systematic review with meta-analysis of cardiovascular outcome trials. Diabetes Res Clin Pr. 2020;162 doi: 10.1016/j.diabres.2020.108114. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert M.P., Bain S.C., Franek E., et al. Effect of liraglutide on cardiovascular outcomes in elderly patients: a post hoc analysis of a randomized controlled trial. Ann Intern Med. 2019;170:423–426. doi: 10.7326/M18-1569. [DOI] [PubMed] [Google Scholar]
- 19.Mentz R.J., Bethel M.A., Merrill P., et al. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL trial. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle M.C., Gerstein H.C., Xavier D., et al. Efficacy and safety of Dulaglutide in older patients: a post hoc analysis of the REWIND trial. J Clin Endocrinol Metab. 2021;106:1345–1351. doi: 10.1210/clinem/dgab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik M.E., Butt J.H., Strange J.E., et al. Initiation of SGLT2 inhibitors and GLP-1 receptor agonists according to level of frailty in people with type 2 diabetes and cardiovascular disease in Denmark: a cross-sectional, nationwide study. Lancet Healthy Longev. 2023;4:e552–e560. doi: 10.1016/S2666-7568(23)00164-2. [DOI] [PubMed] [Google Scholar]
- 22.Dave C.V., Schneeweiss S., Wexler D.J., Brill G., Patorno E. Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013-2018. Diabetes Care. 2020;43:921–924. doi: 10.2337/dc19-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funck K.L., Bjerg L., Isaksen A.A., Sandbaek A., Grove E.L. Gender disparities in time-to-initiation of cardioprotective glucose-lowering drugs in patients with type 2 diabetes and cardiovascular disease: a Danish nationwide cohort study. Cardiovasc Diabetol. 2022;21:279. doi: 10.1186/s12933-022-01713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce J.B., Vaduganathan M., Fonarow G.C., et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: the get with the guidelines-heart failure registry. JAMA Cardiol. 2023;8:652–661. doi: 10.1001/jamacardio.2023.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M., Woodward M., Vaartjes I., et al. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S.R., Everett B.M., Birtcher K.K., et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.