Abstract
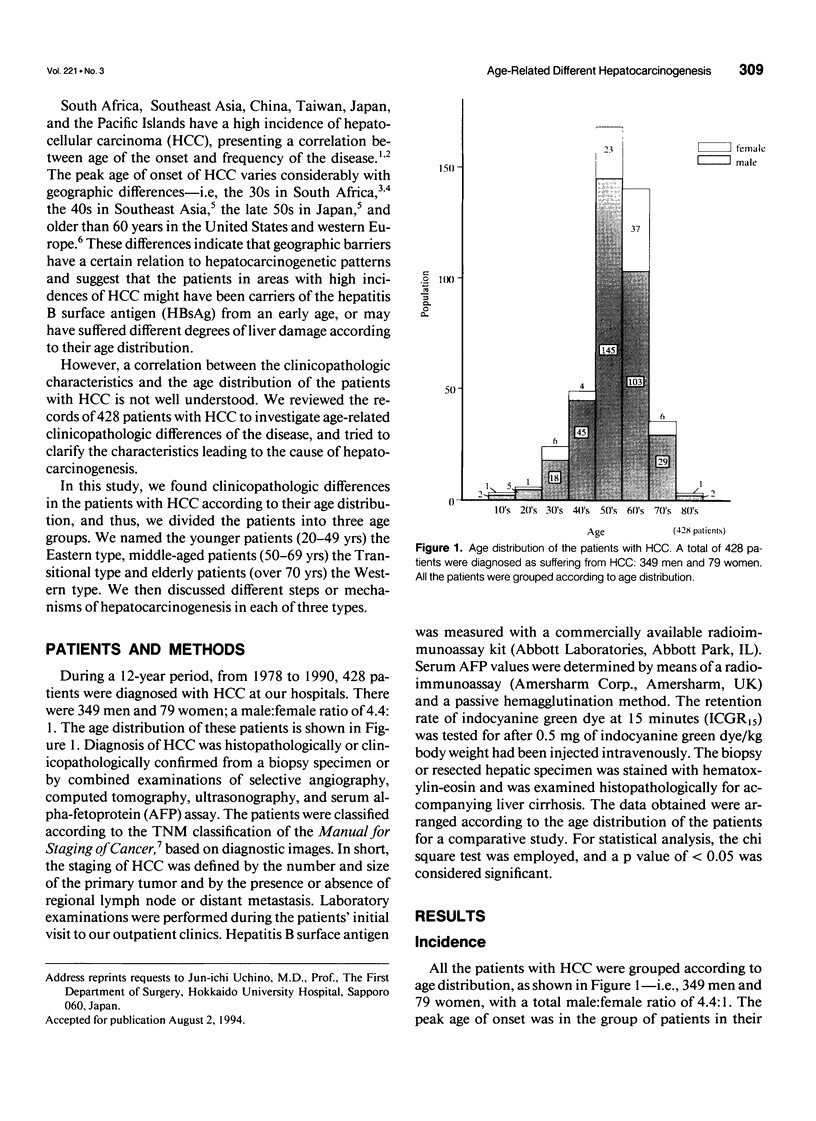
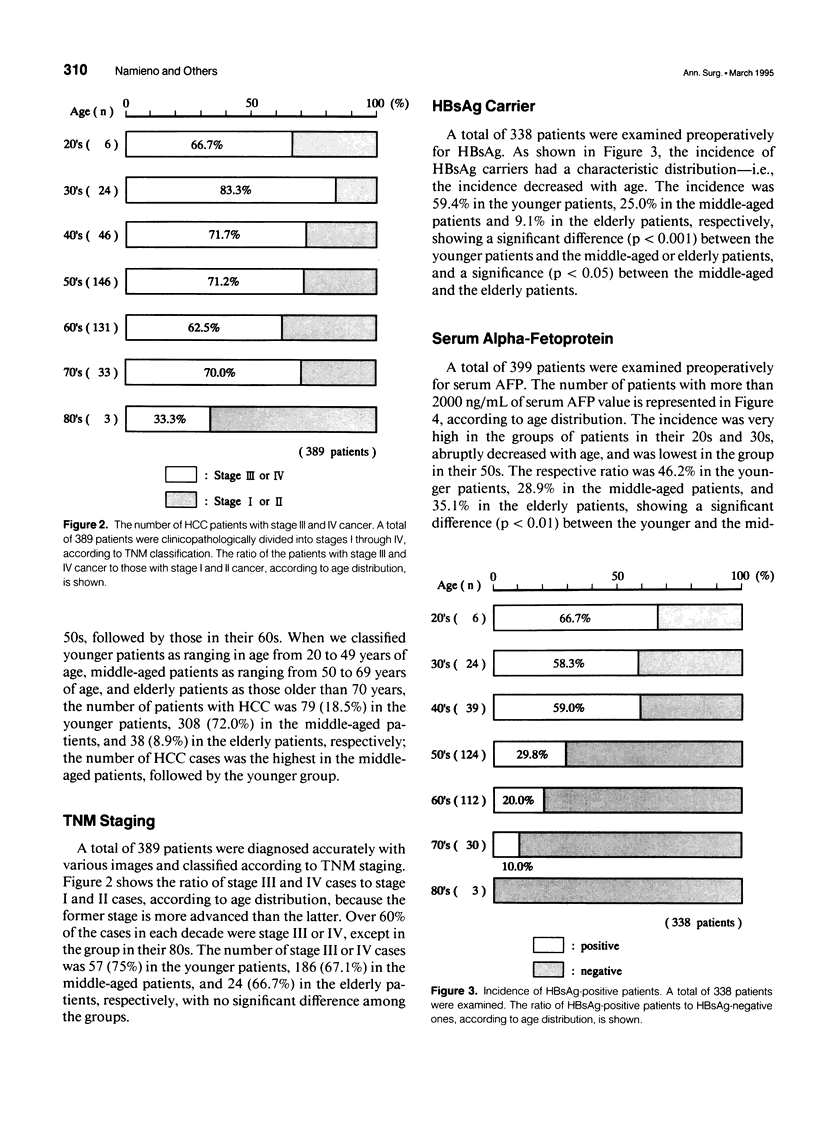
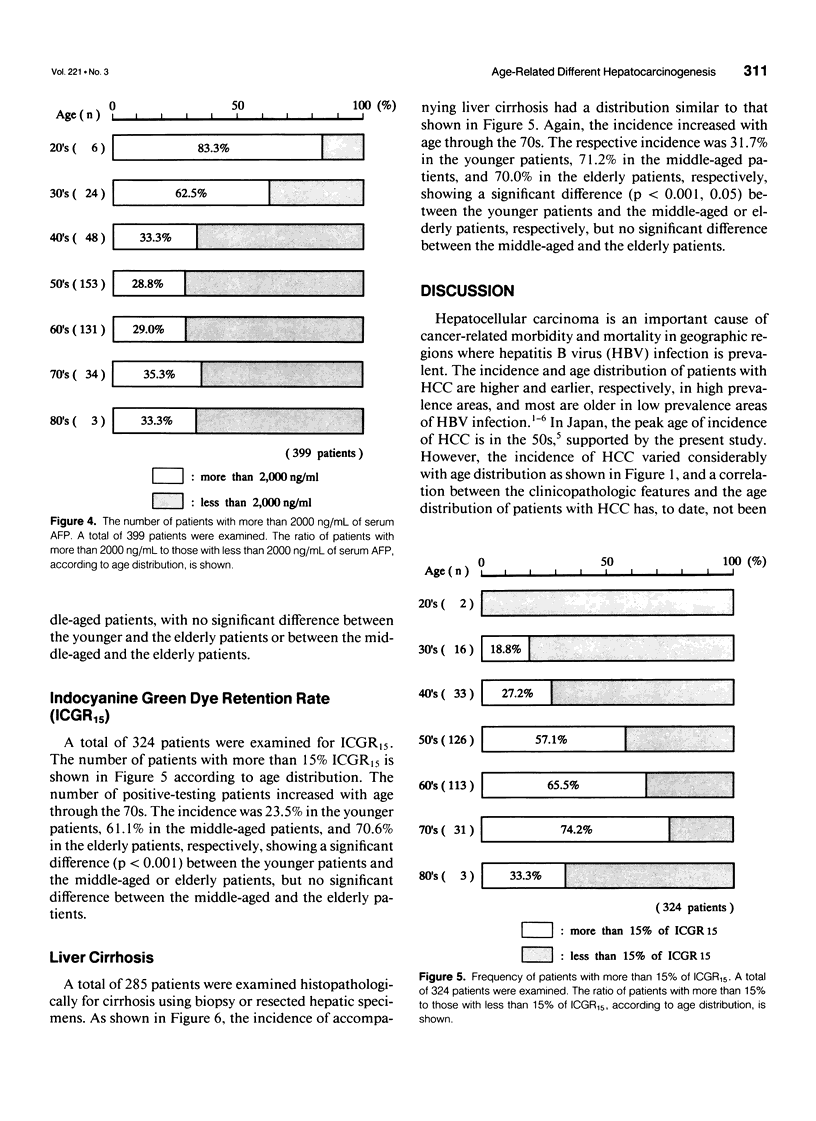
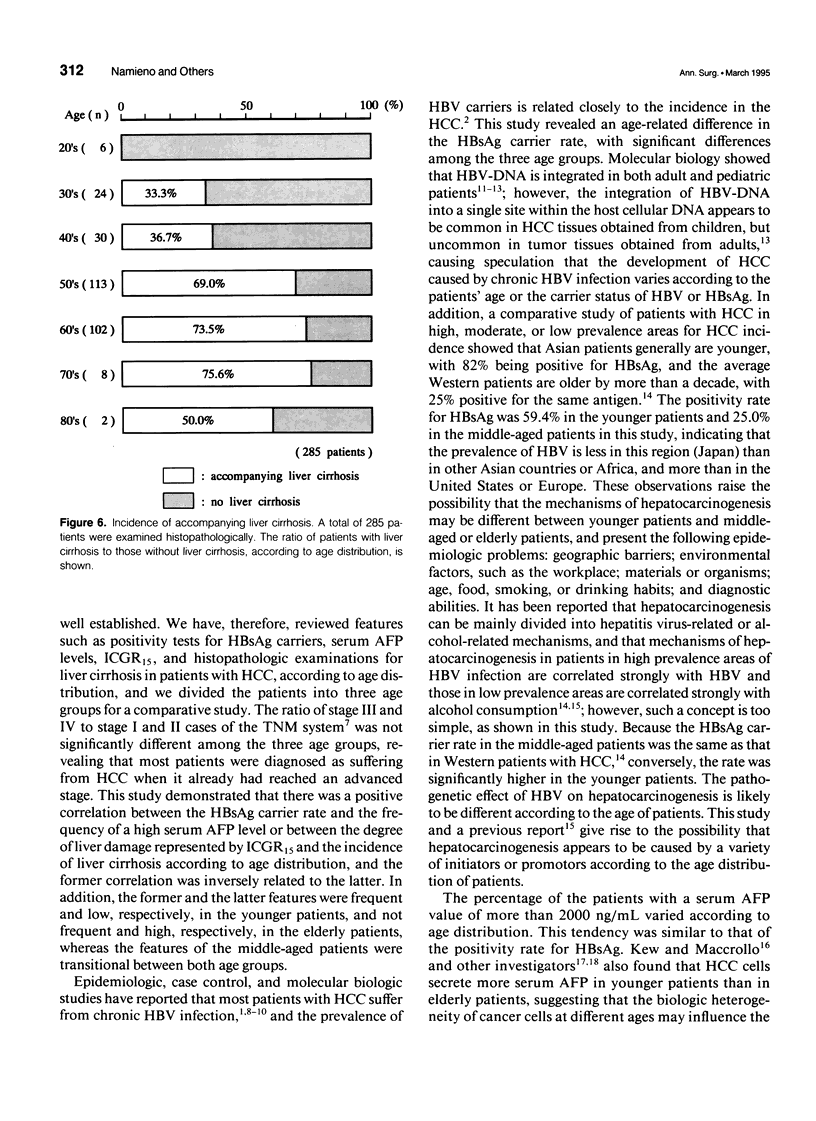
OBJECTIVE: The authors attempted to clarify the clinicopathologic differences of hepatocellular carcinoma (HCC) patients, according to age distribution, and to investigate whether these differences contribute a certain hepatocarcinogenesis. SUMMARY BACKGROUND DATA: Hepatitis-associated viruses causing HCC have been investigated, and the infection of the viruses and etiologically, the peak age of the disease vary according to geographic barriers. However, a correlation between clinicopathologic differences and the age distribution of the patients is not well understood. METHODS: The authors reviewed their institutional experience from 1978 to 1990 in treating 428 patients with HCC. The carrier rate for hepatitis B surface antigen (HBsAg), the frequency of occurrence of high serum alpha-fetoprotein (AFP) of 2000 ng/mL, the degree of liver damage represented by the retention rate of indocyanine green dye at 15 minutes (ICGR15), and the incidence of accompanying liver cirrhosis were investigated and compared in each decade of age. RESULTS: The HBsAg carrier rate and the frequency of high serum AFP values were significantly prominent in the younger patients (20-49 yrs). The degree of liver damage and the incidence of liver cirrhosis were prominent in the elderly patients (older than 70 yrs) or the middle-aged patients (50-69 yrs); however, these four values in the middle-aged patients were intermediate with respect to those observed in the other two age groups. In addition, there was a positive correlation between the HBsAg carrier rate and the frequency of high serum AFP values or between the degree of liver damage represented by ICGR15 and the incidence of liver cirrhosis, showing that the former correlation was inversely related to the latter. CONCLUSIONS: The authors' study indicates that there are age-related differences of clinicopathologic features in HCC patients, suggesting that there are different steps or mechanisms of hepatocarcinogenesis according to the patient's age-distribution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony P. P. Primary carcinoma of the liver: a study of 282 cases in Ugandan Africans. J Pathol. 1973 May;110(1):37–48. doi: 10.1002/path.1711100105. [DOI] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Chang M. H., Chen P. J., Chen J. Y., Lai M. Y., Hsu H. C., Lian D. C., Liu Y. G., Chen D. S. Hepatitis B virus integration in hepatitis B virus-related hepatocellular carcinoma in childhood. Hepatology. 1991 Feb;13(2):316–320. [PubMed] [Google Scholar]
- Chen C. J., Liang K. Y., Chang A. S., Chang Y. C., Lu S. N., Liaw Y. F., Chang W. Y., Sheen M. C., Lin T. M. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991 Mar;13(3):398–406. [PubMed] [Google Scholar]
- Chen D. S., Sung J. L. Serum alphafetoprotein in hepatocellular carcinoma. Cancer. 1977 Aug;40(2):779–783. doi: 10.1002/1097-0142(197708)40:2<779::aid-cncr2820400227>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Dempo K., Chisaka N., Yoshida Y., Kaneko A., Onoé T. Immunofluorescent study on alpha-fetoprotein-producing cells in the early stage of 3'-methyl-4-dimethylaminoazobenzene carcinogenesis. Cancer Res. 1975 May;35(5):1282–1287. [PubMed] [Google Scholar]
- Esteban J. I., Esteban R., Viladomiu L., López-Talavera J. C., González A., Hernández J. M., Roget M., Vargas V., Genescà J., Buti M. Hepatitis C virus antibodies among risk groups in Spain. Lancet. 1989 Aug 5;2(8658):294–297. doi: 10.1016/s0140-6736(89)90485-6. [DOI] [PubMed] [Google Scholar]
- Hino O., Shows T. B., Rogler C. E. Hepatitis B virus integration site in hepatocellular carcinoma at chromosome 17;18 translocation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8338–8342. doi: 10.1073/pnas.83.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew M. C., Geddes E. W. Hepatocellular carcinoma in rural southern African blacks. Medicine (Baltimore) 1982 Mar;61(2):98–108. doi: 10.1097/00005792-198203000-00004. [DOI] [PubMed] [Google Scholar]
- Kew M. C., Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology. 1988 Feb;94(2):439–442. doi: 10.1016/0016-5085(88)90434-9. [DOI] [PubMed] [Google Scholar]
- Liver Cancer Study Group of Japan Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990 Mar;211(3):277–287. [PMC free article] [PubMed] [Google Scholar]
- London W. T. Primary hepatocellular carcinoma--etiology, pathogenesis, and prevention. Hum Pathol. 1981 Dec;12(12):1085–1097. doi: 10.1016/s0046-8177(81)80329-2. [DOI] [PubMed] [Google Scholar]
- Manns M., Meyer zum Büschenfelde K. H. A mitochondrial antigen-antibody system in cholestatic liver disease detected by radioimmunoassay. Hepatology. 1982 Jan-Feb;2(1):1–7. doi: 10.1002/hep.1840020102. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Minase T., Onhoe T. Demonstration of glucose 6-phosphatase activity in the oval cells of rat liver and the significance of the oval cells in azo dye carcinogenesis. Cancer Res. 1974 Dec;34(12):3379–3386. [PubMed] [Google Scholar]
- Okuda K., Kubo Y., Obata H. Serum alpha-fetoprotein in the relatively early stages of hepatocellular carcinoma and its relationship to gross anatomical types. Ann N Y Acad Sci. 1975 Aug 22;259:248–252. doi: 10.1111/j.1749-6632.1975.tb25420.x. [DOI] [PubMed] [Google Scholar]
- Poenaru D., Szilagyi A., Zabad F., Lamoureux E., Lough J. O. Hepatocellular carcinoma: comparison of clinical features among ethnic groups in an area of low prevalence. Am J Gastroenterol. 1991 Apr;86(4):487–494. [PubMed] [Google Scholar]
- Sandler D. P., Sandler R. S., Horney L. F. Primary liver cancer mortality in the United States. J Chronic Dis. 1983;36(3):227–236. doi: 10.1016/0021-9681(83)90057-7. [DOI] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tanaka K., Hirohata T., Koga S., Sugimachi K., Kanematsu T., Ohryohji F., Nawata H., Ishibashi H., Maeda Y., Kiyokawa H. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991 Jun 1;51(11):2842–2847. [PubMed] [Google Scholar]
- Tanaka T., Miyamoto H., Hino O., Kitagawa T., Koike M., Iizuka T., Sakamoto H., Ooshima A. Primary hepatocellular carcinoma with hepatitis B virus-DNA integration in a 4-year-old boy. Hum Pathol. 1986 Feb;17(2):202–204. doi: 10.1016/s0046-8177(86)80296-9. [DOI] [PubMed] [Google Scholar]
- Vogel C. L., Primack A., McIntire K. R., Carbone P. P., Anthony P. P. Serum alpha-fetoprotein in 184 Ugandan patients with hepatocellular carcinoma. Clinical, laboratory, and histopathologic correlations. Cancer. 1974 Apr;33(4):959–964. doi: 10.1002/1097-0142(197404)33:4<959::aid-cncr2820330410>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi H., Yoshida E., Kobayashi M., Koike K. Direct evidence for the expression of integrated hepatitis B virus DNA in human hepatoma tissue. Gan. 1984 Sep;75(9):743–746. [PubMed] [Google Scholar]


