Abstract
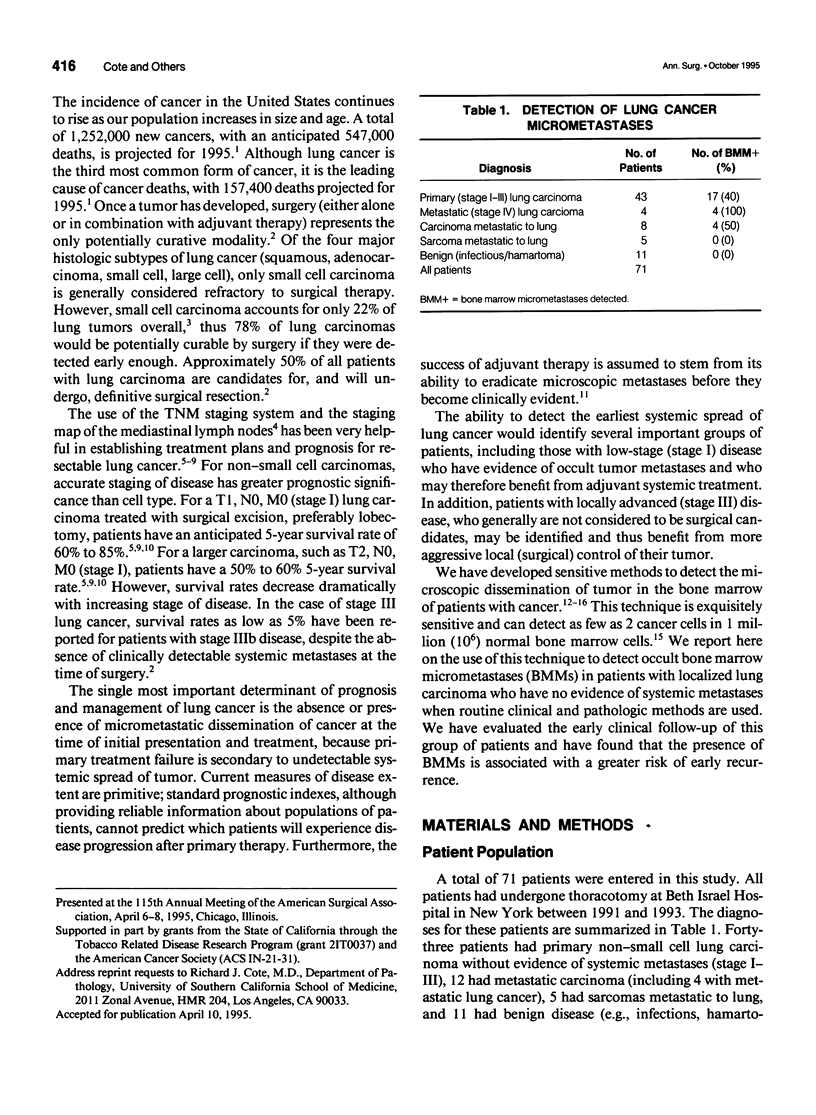
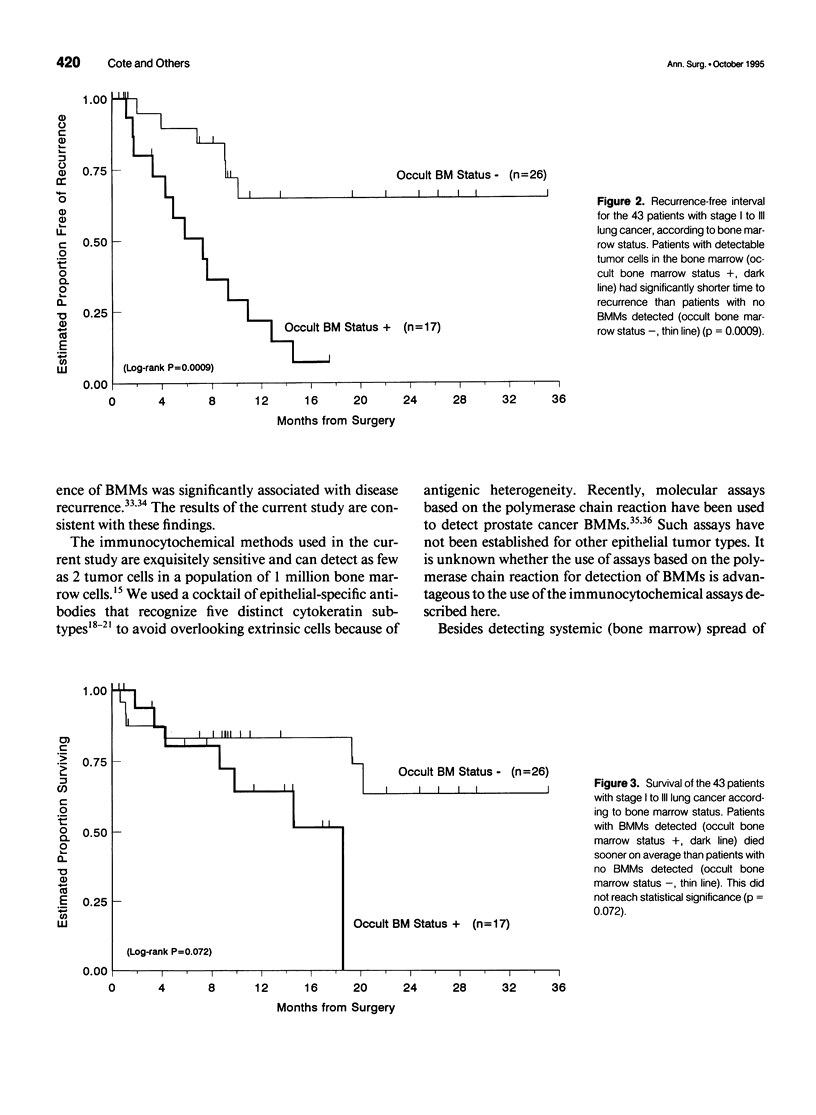
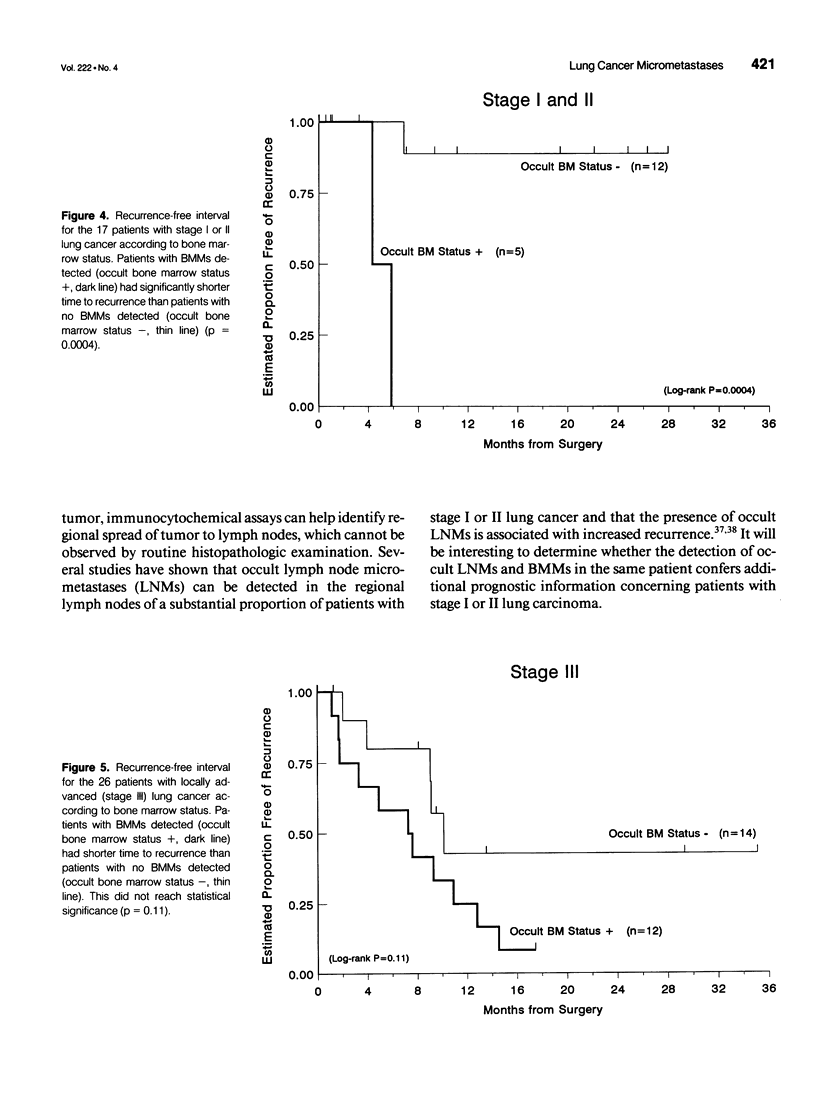
OBJECTIVES: A large proportion of patients with operable lung carcinoma (no evidence of systemic spread of tumor) develop metastatic disease after primary therapy. More sensitive and specific methods are needed to identify patients at highest risk for recurrence who may benefit most from adjuvant therapy, while sparing those patients who do not require such treatment. SUMMARY BACKGROUND DATA: Using epithelial-specific monoclonal antibodies, the authors have developed an immunocytochemical assay capable of detecting as few as 2 lung cancer cells in 1 million bone marrow cells. METHODS: The assay was used to test the bone marrow (from resected ribs) of 43 patients with primary non-small cell lung carcinoma who showed no clinical or pathologic evidence of systemic disease. RESULTS: Occult bone marrow micrometastases (BMMs) were detected in 40% of patients (17/43) with non-small cell lung cancer, including 29% (5/17) of patients with stage I or II disease and 46% of whom (12/26) had stage III disease. The median follow-up was 13.6 months. Patients with occult BMMs had significantly shorter times to disease recurrence compared with patients without BMMs (7.3 vs. > 35.1 months, p = 0.0009). Furthermore, for patients with stage I or II disease, the presence of occult BMMs was significantly associated with a higher rate of recurrence (p = 0.0004). CONCLUSIONS: The detection of occult BMMs identifies patients with operable non-small cell lung carcinoma who are at significantly increased risk for recurrence, independent of tumor stage, and may be useful in evaluating patients for adjuvant treatment protocols.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobrow L. G., Makin C. A., Law S., Bodmer W. F. Expression of low molecular weight cytokeratin proteins in cervical neoplasia. J Pathol. 1986 Feb;148(2):135–140. doi: 10.1002/path.1711480203. [DOI] [PubMed] [Google Scholar]
- Chen Z. L., Perez S., Holmes E. C., Wang H. J., Coulson W. F., Wen D. R., Cochran A. J. Frequency and distribution of occult micrometastases in lymph nodes of patients with non-small-cell lung carcinoma. J Natl Cancer Inst. 1993 Mar 17;85(6):493–498. doi: 10.1093/jnci/85.6.493. [DOI] [PubMed] [Google Scholar]
- Cote R. J., Rosen P. P., Hakes T. B., Sedira M., Bazinet M., Kinne D. W., Old L. J., Osborne M. P. Monoclonal antibodies detect occult breast carcinoma metastases in the bone marrow of patients with early stage disease. Am J Surg Pathol. 1988 May;12(5):333–340. doi: 10.1097/00000478-198805000-00001. [DOI] [PubMed] [Google Scholar]
- Cote R. J., Rosen P. P., Lesser M. L., Old L. J., Osborne M. P. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991 Oct;9(10):1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- Dearnaley D. P., Ormerod M. G., Sloane J. P. Micrometastases in breast cancer: long-term follow-up of the first patient cohort. Eur J Cancer. 1991;27(3):236–239. doi: 10.1016/0277-5379(91)90504-7. [DOI] [PubMed] [Google Scholar]
- Diel I. J., Kaufmann M., Goerner R., Costa S. D., Kaul S., Bastert G. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis. J Clin Oncol. 1992 Oct;10(10):1534–1539. doi: 10.1200/JCO.1992.10.10.1534. [DOI] [PubMed] [Google Scholar]
- Hansen H. H., Muggia F. M., Selawry O. S. Bone-marrow examination in 100 consecutive patients with bronchogenic carcinoma. Lancet. 1971 Aug 28;2(7722):443–445. doi: 10.1016/s0140-6736(71)92622-5. [DOI] [PubMed] [Google Scholar]
- Hansen H. H., Muggia F. M. Staging of inoperable patients with bronchogenic carcinoma with special reference to bone marrow examination and peritoneoscopy. Cancer. 1972 Nov;30(5):1395–1401. doi: 10.1002/1097-0142(197211)30:5<1395::aid-cncr2820300538>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Klein E., Vánky F., Galili U., Vose B. M., Fopp M. Separation and characteristics of tumor-infiltrating lymphocytes in man. Contemp Top Immunobiol. 1980;10:79–107. doi: 10.1007/978-1-4684-3677-8_4. [DOI] [PubMed] [Google Scholar]
- Leonard R. C., Duncan L. W., Hay F. G. Immunocytological detection of residual marrow disease at clinical remission predicts metastatic relapse in small cell lung cancer. Cancer Res. 1990 Oct 15;50(20):6545–6548. [PubMed] [Google Scholar]
- Lindemann F., Schlimok G., Dirschedl P., Witte J., Riethmüller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992 Sep 19;340(8821):685–689. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi J. L., Berger U., Easton D., McDonnell T., Redding W. H., Gazet J. C., McKinna A., Powles T. J., Coombes R. C. Micrometastases in bone marrow in patients with primary breast cancer: evaluation as an early predictor of bone metastases. Br Med J (Clin Res Ed) 1987 Oct 31;295(6606):1093–1096. doi: 10.1136/bmj.295.6606.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini N., Beattie E. J., Jr Results of surgical treatment in Stage I lung cancer. J Thorac Cardiovasc Surg. 1977 Oct;74(4):499–505. [PubMed] [Google Scholar]
- Martini N., Flehinger B. J., Nagasaki F., Hart B. Prognostic significance of N1 disease in carcinoma of the lung. J Thorac Cardiovasc Surg. 1983 Nov;86(5):646–653. [PubMed] [Google Scholar]
- Martini N., Flehinger B. J., Zaman M. B., Beattie E. J., Jr Results of resection in non-oat cell carcinoma of the lung with mediastinal lymph node metastases. Ann Surg. 1983 Sep;198(3):386–397. doi: 10.1097/00000658-198309000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed M. R., Flehinger B. J., Zaman M. B., Heelan R. T., Perchick W. A., Martini N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest. 1984 Jul;86(1):44–53. doi: 10.1378/chest.86.1.44. [DOI] [PubMed] [Google Scholar]
- Moreno J. G., Croce C. M., Fischer R., Monne M., Vihko P., Mulholland S. G., Gomella L. G. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer Res. 1992 Nov 1;52(21):6110–6112. [PubMed] [Google Scholar]
- Muggia F. M., Chervu L. R. Lung cancer: diagnosis in metastatic sites. Semin Oncol. 1974 Sep;1(3):217–228. [PubMed] [Google Scholar]
- Osborne M. P., Asina S., Wong G. Y., Old L. J., Cote R. J. Immunofluorescent monoclonal antibody detection of breast cancer in bone marrow: sensitivity in a model system. Cancer Res. 1989 May 1;49(9):2510–2513. [PubMed] [Google Scholar]
- Pantel K., Izbicki J. R., Angstwurm M., Braun S., Passlick B., Karg O., Thetter O., Riethmüller G. Immunocytological detection of bone marrow micrometastasis in operable non-small cell lung cancer. Cancer Res. 1993 Mar 1;53(5):1027–1031. [PubMed] [Google Scholar]
- Passlick B., Izbicki J. R., Kubuschok B., Nathrath W., Thetter O., Pichlmeier U., Schweiberer L., Riethmüller G., Pantel K. Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small-cell lung cancer. J Clin Oncol. 1994 Sep;12(9):1827–1832. doi: 10.1200/JCO.1994.12.9.1827. [DOI] [PubMed] [Google Scholar]
- Ramsdell J. W., Peters R. M., Taylor A. T., Jr, Alazraki N. P., Tisi G. M. Multiorgan scans for staging lung cancer. Correlation with clinical evaluation. J Thorac Cardiovasc Surg. 1977 May;73(5):653–659. [PubMed] [Google Scholar]
- Redding W. H., Coombes R. C., Monaghan P., Clink H. M., Imrie S. F., Dearnaley D. P., Ormerod M. G., Sloane J. P., Gazet J. C., Powles T. J. Detection of micrometastases in patients with primary breast cancer. Lancet. 1983 Dec 3;2(8362):1271–1274. doi: 10.1016/s0140-6736(83)91150-9. [DOI] [PubMed] [Google Scholar]
- Rosenow E. C., 3rd, Carr D. T. Bronchogenic carcinoma. CA Cancer J Clin. 1979 Jul-Aug;29(4):233–245. doi: 10.3322/canjclin.29.4.233. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr Rationale for adjuvant chemotherapy. Cancer. 1977 Jun;39(6 Suppl):2875–2882. doi: 10.1002/1097-0142(197706)39:6<2875::aid-cncr2820390675>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Schlimok G., Funke I., Holzmann B., Göttlinger G., Schmidt G., Häuser H., Swierkot S., Warnecke H. H., Schneider B., Koprowski H. Micrometastatic cancer cells in bone marrow: in vitro detection with anti-cytokeratin and in vivo labeling with anti-17-1A monoclonal antibodies. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8672–8676. doi: 10.1073/pnas.84.23.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo D. V., Michie S. A., Crabtree G. S., Warnke R. A., Rouse R. V. Monoclonal anti-keratin (AE1) reactivity in routinely processed tissue from 166 human neoplasms. Am J Clin Pathol. 1985 Dec;84(6):697–704. doi: 10.1093/ajcp/84.6.697. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Pairolero P. C., Davis C. S., Bernatz P. E., Payne W. S., Taylor W. F., Uhlenhopp M. A., Fontana R. S. Survival of patients surgically treated for stage I lung cancer. J Thorac Cardiovasc Surg. 1981 Jul;82(1):70–76. [PubMed] [Google Scholar]
- Wingo P. A., Tong T., Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995 Jan-Feb;45(1):8–30. doi: 10.3322/canjclin.45.1.8. [DOI] [PubMed] [Google Scholar]
- Wood D. P., Jr, Banks E. R., Humphreys S., Rangnekar V. M. Sensitivity of immunohistochemistry and polymerase chain reaction in detecting prostate cancer cells in bone marrow. J Histochem Cytochem. 1994 Apr;42(4):505–511. doi: 10.1177/42.4.7510319. [DOI] [PubMed] [Google Scholar]



