Figure 5.
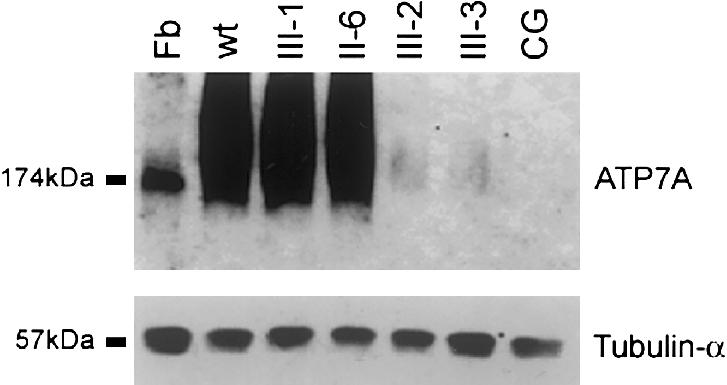
Western blot analysis of ATP7A levels. Protein from lymphoblastoid cell lines from CG, II-6, III-1, III-2, and III-3 was isolated according to a procedure described by Ambrosini and Mercer (1999). Fibroblast (Fb) protein from an unrelated normal control cell line was isolated according to a procedure described by Dierick et al. (1997). One hundred micrograms of protein per lane was fractionated through a 7.5% acrylamide gel and then was electroblotted onto a nitrocellulose membrane. This membrane was then hybridized with a 1:1,000 dilution of polyclonal α-ATP7A (upper panel), as described by Dierick et al. (1997); after several washes in Tris buffered saline solution plus Triton X-20, this membrane was incubated with a 1:2,000 dilution of horseradish peroxidase–conjugated donkey anti-rabbit antibodies. α-ATP7A hybridized to a 174-kD protein in fibroblast, consistent with ATP7A. Hybridization to a 174-kD protein in the fibroblast cell line confirms that the ATP7A antibody is hybridizing to the correct protein from lymphoblastoid cells. To demonstrate loading consistency, the blot was hybridized with a 1:10,000 dilution of tubulin-α Ab-2 antibody, which hybridized to a 57-kD protein. Ambrosini and Mercer (1999) have reported smearing of ATP7A isolated from lymphoblastoid cell lines and have suggested that it most likely was a result of protein glycosylation.
