Abstract
Introduction
In vitro, clascoterone inhibits androgen-induced sebum production—a key driver of acne pathogenesis—although the exact mechanism of action of clascoterone for the treatment of acne is unknown. This study evaluated reductions in casual sebum production following 12 weeks of clascoterone cream 1% treatment in patients with acne.
Methods
Patients ≥ 12 years old with mild-to-moderate acne applied clascoterone cream 1% twice daily for 12 weeks. The primary endpoint was the reduction in casual sebum measurements at Week 12. Additional endpoints included Investigator’s Global Assessment (IGA) score, inflammatory and noninflammatory lesion counts (ILC and NILC), and tolerability. Data were analyzed using Student’s t-test and Wilcoxon signed-rank test.
Results
Forty patients with a mean age of 20.9 years were enrolled, all of whom completed Week 12. Significant percentage reductions from baseline were observed in sebum measurements (27%), ILC (54%), and NILC (34%; all p < 0.001), and patients achieved a 29% reduction in IGA score. No tolerability or safety issues were identified during the 12-week interim analysis period.
Conclusion
Clascoterone cream 1% led to significant reductions in sebum measurements with improvements in acne severity and was well tolerated.
Trial Registration
ClinicalTrials.gov identifier, NCT06415279.
Keywords: Clascoterone, Topical, Acne vulgaris, Sebum, Efficacy, Tolerability
Key Summary Points
| Why carry out this study? |
| Androgen-induced excess sebum production by the sebaceous glands is an important early step in the pathophysiology of acne. |
| Clascoterone cream 1% decreases androgen-stimulated sebum production in vitro; however, the exact mechanism of action of clascoterone cream 1% for the treatment of acne is unknown. |
| This study aimed to demonstrate the effect of clascoterone cream 1% on facial casual sebum production as well as acne severity in patients with acne vulgaris. |
| What was learned from the study? |
| Significant reductions from baseline were observed in sebum measurements, Investigator’s Global Assessment score, lesion counts, and subjective facial characteristics (oily appearance, pore size, and shine) following treatment with clascoterone cream 1%, and no tolerability or safety issues were identified. |
| This study shows that clascoterone cream 1% decreases casual sebum production and provides further evidence of its efficacy as a topical option for treating androgen-stimulated excess sebum production in male and female patients with acne. |
Introduction
Acne vulgaris is a chronic inflammatory skin disorder with a substantial global disease burden, particularly among adolescents and young adults [1]. The pathophysiology of acne begins with androgen-induced sebum production by sebaceous glands [2]. Sebum provides a favorable environment for the proliferation of Cutibacterium acnes, resulting in inflammatory responses that drive the formation of papules, pustules, and other acne lesions [2]. Medications that target sebaceous glands, such as oral isotretinoin and systemic hormonal agents (e.g., spironolactone), show efficacy in patients with acne; however, these medications are typically reserved for moderate-to-severe acne, may not be appropriate for both male and female patients, and are limited by other safety concerns [3].
Topical therapies are often the mainstay of initial treatment for mild-to-moderate acne [3]. Commonly used topical medications include topical retinoids, topical antibiotics, benzoyl peroxide, salicylic acid, azelaic acid, and clascoterone [3]. Topical retinoids, topical antibiotics, salicylic acid, and azelaic acid primarily treat acne through comedolytic, antibacterial, and/or anti-inflammatory effects, whereas benzoyl peroxide is an antimicrobial agent with mild comedolytic properties [3]; none of these agents are shown to decrease sebum production. The efficacy of clascoterone cream 1%, an androgen receptor inhibitor, for the treatment of acne vulgaris is primarily attributed to the inhibition of androgen-mediated sebum production based on evidence from in vitro studies [3, 4]. However, the effect of clascoterone cream 1% on sebum production in patients with acne has not been clinically demonstrated.
The objective of this study was to demonstrate the effect of clascoterone cream 1% on facial sebum production using sebumeter measurements and its efficacy using other investigator-assessed measures. The 12-week interim results from a 52-week study to evaluate facial sebum production following treatment with clascoterone cream 1% in patients with mild-to-moderate acne are presented.
Methods
Study Design and Patients
The study was conducted in accordance with Good Clinical Practice guidelines and all local legal and regulatory requirements and was registered with ClinicalTrials.gov (NCT06415279). Institutional review board (Allendale Institutional Review Board, Old Lyme, CT) approval was obtained for the study protocol. All patients (and/or a legally acceptable representative, as applicable) were required to provide written informed consent to participate in the study.
This single-site study enrolled male and nonpregnant female patients ≥ 12 years old with mild-to-moderate acne. Patients with all Fitzpatrick skin types were eligible. Key inclusion criteria were 10–100 total noninflammatory lesions, 10–50 total inflammatory lesions, no cysts, and ≤ 2 nodules on the face. Eligible patients agreed to use only clascoterone cream 1% twice daily for acne treatment during the study period and were not permitted to use other medicated cleansers, moisturizers, or acne treatments or to introduce any new colored cosmetics or skin care products (e.g., lipstick, eye shadow, foundation, blush) during the study. Patients were required to avoid sun exposure (or use sunscreen if sun exposure was unavoidable) and refrain from professional or facial spa procedures during the study.
Key exclusion criteria included (1) any dermatological disorder other than acne determined to interfere with the accurate evaluation of the patient’s skin characteristics; (2) any surgery or invasive medical procedure planned during the study; (3) observable suntan, scars, nevi, tattoos, excessive hair, or other dermatological conditions on the face that could interfere with study assessments; (4) current or planned use of prescription antibiotics, inhaled steroids, hormones, or other medications within specified time ranges that could interfere with study results (Table 1); (5) prescription for medications or retinoids for acne treatment, ongoing dermatologist care for acne, or current acne flare; (6) initiation of hormone replacement therapies or hormonal contraceptive agents < 3 months prior to study enrollment.
Table 1.
Prohibited medications and washout periods
| Medication | Washout period |
|---|---|
| Prescription (oral or topical) antibiotics, inhaled steroids (except those prescribed for allergies), hormones (e.g., pre- or post-menopausal HRT, insulin), or other medications that could affect skin sensitivity | 1 month prior to Visit 1 |
| Any prescription medication for acne | 1 month prior to Visit 1 |
| Topical prescription retinoids | 1 month prior to Visit 1 |
| Isotretinoin or other oral retinoids | 6 months prior to Visit 1 |
| Any of the following on the face | 2 weeks prior to Visit 1 |
| Light therapy | |
| Over-the-counter topical medications or products other than sunscreen, including antiacne or antibacterial agents, anti-inflammatory agents, and retinoids |
HRT hormone replacement therapy
Treatments
All enrolled patients applied clascoterone cream 1% to the entire face twice daily. Patients were permitted to continue using their self-selected skincare products as long as they had been used for at least 30 days prior to enrollment. Concomitant use of additional medicated cleansers, moisturizers, or acne treatments was not permitted. Patients were instructed to wash their faces 2–4 h prior to each study visit and not to apply any topical facial products until the completion of the visit.
Assessments
All assessments were performed at baseline and every 2 weeks during the treatment period through Week 12. The primary efficacy endpoint was a reduction in casual sebum measurements from the forehead obtained with a sebumeter. The casual sebum level is a measurement of the lipid content present on the skin surface after remaining unwashed for several hours [5]. Patients were instructed to wash their face 2–4 h prior to their visit and were not to apply any topical facial product(s) until the completion of the visit. Three sebum measurements were taken from the right, center, and left forehead 2–4 h after face washing at each study visit. The conditions under which sebum measurements were performed were kept consistent between each visit. All patients acclimated to the room for 20 min prior to the sebum measurements. Humidity was maintained at 40% to 50%, and temperature was maintained at 70°F–72°F. All measurements were performed by the same investigator at each visit.
Additional efficacy endpoints included oily appearance, pore size, and facial shine, which were assessed by the investigator on a 5-point scale (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe), improvement in Investigator’s Global Assessment (IGA) score, and reductions in inflammatory and noninflammatory lesion counts (ILC and NILC, respectively). The IGA was rated on a 5-point scale from 0 (clear) to 4 (severe) at each visit.
Safety assessments included frequency and severity of adverse events and tolerability assessments. At each visit, the investigator assessed the severity of peeling, dryness, redness, and swelling on a 5-point ordinal scale from 0 (none) to 4 (severe), and patients rated the severity of stinging, itching, and burning on a scale from 0 (none) to 4 (severe).
Statistical Analyses
No formal sample size calculations were performed because of the exploratory nature of this study. Tolerability assessments and IGA scores were analyzed using the Wilcoxon signed-rank test. Sebumeter measurements and reductions in lesion counts were analyzed using Student’s t-test, and p ≤ 0.05 was accepted as significant with no adjustment for multiple comparisons. No transformations were applied to the data. Statistical analyses included all enrolled patients.
Results
Patient Demographics and Baseline Characteristics
A total of 40 patients with a mean age of 20.9 years were enrolled (Table 2), all of whom completed Week 12. Twenty-four (60%) patients were female, and the majority (63%) were White. Patients entered the study with mild (IGA score of 2; 57.5%) or moderate (IGA score of 3; 42.5%) acne and had a mean ± standard deviation (SD) sebumeter reading of 115.9 ± 50.5 at baseline.
Table 2.
Summary of demographics and baseline characteristics
| Category | Clascoterone cream 1%, N = 40 |
|---|---|
| Age, years, mean | 20.9 |
| Range (min–max) | 12–58 |
| Sex, n (%) | |
| Male | 16 (40) |
| Female | 24 (60) |
| Race/ethnicity, n (%) | |
| White | 25 (63) |
| Black or African American | 11 (28) |
| Hispanic | 4 (10) |
| Fitzpatrick skin type, n (%) | |
| I | 10 (25) |
| II | 14 (35) |
| III | 5 (13) |
| IV | 4 (10) |
| V | 4 (10) |
| VI | 3 (8) |
| Sebumeter reading, mean ± SD | 115.9 ± 50.5 |
| IGA score, % | |
| 2 | 57.5 |
| 3 | 42.5 |
| ILC, mean ± SD | 16.8 ± 6.3 |
| Papules | 15.2 ± 5.4 |
| Pustules | 1.6 ± 2.0 |
| NILC, mean ± SD | 22.1 ± 10.5 |
| Closed comedones | 20.6 ± 8.5 |
| Open comedones | 1.5 ± 4.0 |
IGA Investigator’s Global Assessment, ILC inflammatory lesion count, NILC noninflammatory lesion count, SD standard deviation
Sebum Production
From baseline to Week 12, the primary efficacy endpoint of mean ± SD sebumeter measurement decreased from 115.9 ± 50.5 to 84.8 ± 37.3, an overall 27% reduction in casual sebum levels (p < 0.001; Fig. 1). Statistically significant reductions from baseline in sebumeter measurements were observed as early as Week 6 of treatment with a mean percent reduction of 22% (p = 0.002), although some fluctuations in sebumeter measurements were observed between visits (Fig. 1).
Fig. 1.
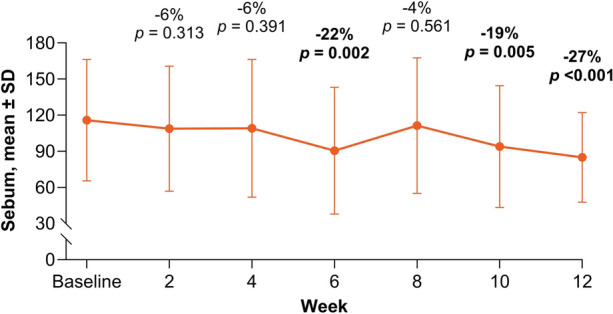
Mean ± SD sebum measurements from baseline to Week 12. Data labels show the percent change compared with baseline. Bold values indicate statistical significance (p < 0.05); SD standard deviation
IGA and Lesion Counts
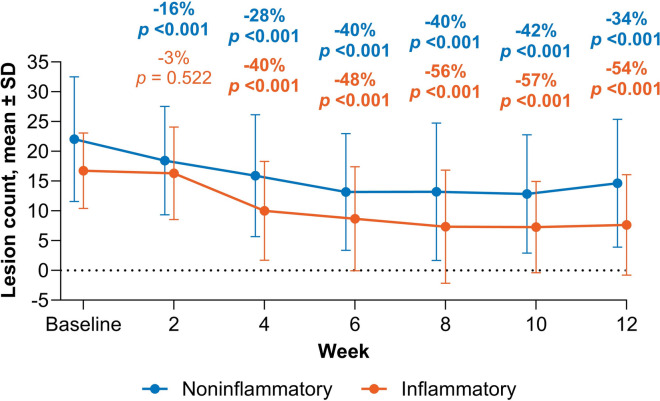
Acne severity as assessed by IGA score improved continuously from baseline to Week 12 based on both score distribution and mean percentage change from baseline (Fig. 2). At Week 12, patients using clascoterone cream 1% had a statistically significant improvement in IGA score with a mean reduction of 29% (p < 0.001 vs baseline; Fig. 2). There were also statistically significant percentage reductions from baseline in NILC and ILC beginning at Week 2 and Week 4, respectively, which continued through Week 12. Treatment with clascoterone cream 1% resulted in a 34% reduction in NILC and 54% reduction in ILC at Week 12 (both p < 0.001 vs baseline; Fig. 3).
Fig. 2.

Percentage of patients with each IGA score from baseline to Week 12. Data are presented as frequency (%). Data labels show the percent change compared with baseline. Bold values indicate statistical significance (p < 0.05); IGA Investigator’s Global Assessment
Fig. 3.
Mean ± SD reductions in ILC and NILC from baseline to Week 12. Data labels show the percent change compared with baseline. Bold values indicate statistical significance (p < 0.05); ILC inflammatory lesion count, NILC noninflammatory lesion count, SD standard deviation
Investigator-Assessed Efficacy Outcomes
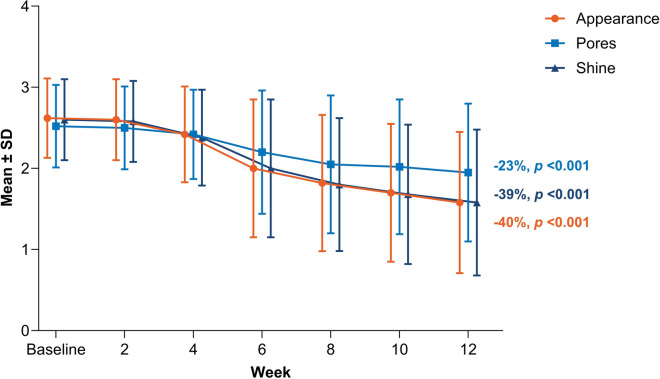
Consistent with the observed decrease in sebum production and improvements in acne severity, there were statistically significant improvements in investigator-assessed facial characteristics including oily appearance and facial shine by Week 4 and pore size by Week 6 (all p < 0.01), with continued improvement through Week 12 (Fig. 4). From baseline to Week 12, patients had a 40% improvement in facial appearance, 23% improvement in pore appearance, and 39% improvement in facial shine (all p < 0.001; Fig. 4).
Fig. 4.
Mean reductions in facial appearance, pore size, and shine from baseline to Week 12. Data labels show the mean percent change relative to baseline. Assessments were made on a 5-point scale from 0 (none) to 4 (severe); SD standard deviation
Tolerability and Safety
No statistically significant changes in tolerability assessments performed by the investigator or patient were observed between baseline and Week 12 (all p > 0.05). Overall, no occurrences of peeling, dryness, redness, or swelling were recorded by the investigator throughout the 12-week treatment period. Similarly, stinging, burning, and itching were rated as none or minimal by patients through Week 12. As of the Week 12 interim analysis time point, no adverse events or experiences were reported.
Discussion
This study provides initial clinical evidence of reductions in measured facial sebum production following treatment with clascoterone cream 1% in patients with acne. Although some systemic treatments are shown to decrease sebum production, there appear to be no other reports demonstrating this for a US Food and Drug Administration (FDA)–approved topical acne medication [6]. The clinical findings from this study recapitulate those reported from the Phase 3 clinical trials, including reductions in the numbers of noninflammatory and inflammatory lesions following 12 weeks of treatment with clascoterone cream 1% [7], and confirm the effect of clascoterone treatment on sebum production. Overall, this study expands the clinical profile of clascoterone cream 1% and helps to illuminate the mechanism of action of clascoterone in the treatment of acne.
Consistent with reductions in objective facial sebum measurements, patients using clascoterone cream 1% had significant improvements in investigator-assessed subjective facial characteristics related to sebum production, including oily appearance, shine, and pore size. Several factors including sebum production, skin elasticity, and the presence of acne may impact pore characteristics [8, 9]. Excessive sebum production is strongly implicated as a contributing factor to increased visible pore quantity and/or size, particularly in people with acne [8, 9], corroborating the association between sebum levels and pore size observed in the current study. Therefore, the clinical benefits of clascoterone cream 1% include improvements in subjective facial characteristics associated with excess sebum production, which are often reported as concerns in individuals with acne, as well as reductions in measured facial sebum levels [10, 11].
Excessive sebum production is a substantial driver of the acne pathogenesis cascade. Other topical agents approved by the FDA for acne treatment act primarily by modulating keratinocyte differentiation and/or antibacterial and anti-inflammatory actions [3] but do not act directly on sebaceous gland activity. Systemic acne medications that reduce sebum production include hormonal therapies and oral isotretinoin [6]. The reductions in sebum measurements reported in this study are consistent with those reported following treatment with systemic antiandrogens in patients with acne. In an open-label trial, treatment with an oral contraceptive led to reductions in sebum measurements from baseline by 15% after two treatment cycles (8 weeks) and 20% after four treatment cycles (16 weeks) in 177 female patients with acne [12]. Reductions in forehead sebum production by 30% were reported following treatment with an oral contraceptive in 41 patients with acne in a randomized, double-blind study [13], with other studies reporting similar results [14, 15].
Previous studies report a wide range of sebum reduction in patients with acne treated with oral isotretinoin. In a prospective study including 35 patients with acne who were treated with oral isotretinoin for 6 months, patients had an overall 36% decline in sebum levels [16], whereas overall percentage reductions of 50% to 75% or greater were reported following isotretinoin therapy in other, similar studies [17–19]. The wide range in reported values may be due to differences in patient characteristics, isotretinoin dosage, duration of treatment at the time of sebum assessments, and method of evaluating sebum production. The reduction in sebum measurements observed in this study following 12 weeks of treatment with clascoterone cream 1%, a topical agent, falls slightly below the range of values reported following 3–7 months of treatment with oral isotretinoin [16–19]. Isotretinoin is also associated with reports of dry lips/skin that are not observed with clascoterone cream 1% [20]. It will be instructive to compare the percentage reductions in sebum values following ≥6 months of treatment with clascoterone cream 1% with those reported for oral isotretinoin.
This study had several potential limitations. Due to the open-label, non-comparative design of the study, it is difficult to attribute the observed effects solely to use of clascoterone cream 1%. However, participants were not permitted to initiate any other skincare products or medications, including prescription and over-the-counter treatments, cleansers, moisturizers, and cosmetics, while participating in the study. Additionally, although the sample size was somewhat small, the data were internally consistent and supported the use of appropriate tests for statistical significance. Although the sebumeter is a validated method commonly used in cosmetic and medical research to quantify changes in casual sebum levels on the skin [21], readings can be affected by external variables. The research center attempted to perform sebum measurements for all patients at the same time of day and under standard environmental conditions (e.g., temperature, humidity) to ensure consistency; however, sebum measurements may have been impacted by the thoroughness of face washing prior to each visit, or other external factors such as fluctuations in weather. Sebumeter measurements can also be user dependent, and the same investigator performed all measurements at each visit to minimize this source of variability. Despite these measures, fluctuations in sebumeter measurements were observed between visits during the study; however, the overall trend indicates that clascoterone cream 1% treatment does decrease sebum production.
Interestingly, improvements in the severity of acne as determined by the reduction in acne lesions preceded the observed decrease in sebum measurements. This may be due to several factors, including fluctuations in sebum measurements and the potential contribution of other pathogenic factors (e.g., reduction in inflammation, microbiome changes) to the observed lesion count reductions. The full 52-week study will also explore changes in the facial microbiome following treatment with clascoterone cream 1%, which will further expand our understanding of the mechanism of action of clascoterone in the treatment of acne vulgaris.
Conclusion
Addressing excessive sebum production is an important component of effective acne treatment. Clascoterone cream 1% provides a topical treatment option for targeting androgen-mediated excessive sebum production that can be used safely in both male and female patients.
Acknowledgements
We thank the participants of the study. The authors also thank Sveta Weiner for her contribution to reviewing the data and statistical analyses in the manuscript.
Medical Writing, Editorial, and Other Assistance
Manuscript preparation and editorial assistance were provided by Dana Lengel, PhD, of Red Nucleus and funded by Sun Pharma.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Zoe D. Draelos. Zoe D. Draelos, Kizito Kyeremateng, and Nicholas Squittieri contributed to drafting or revising the manuscript for critically important intellectual content. All authors read and approved the final manuscript.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Sun Pharma.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of Interest
Zoe D. Draelos was an investigator on this study and received a grant from Sun Pharma. She is also an editorial board member of Dermatology and Therapy. Zoe D. Draelos was not involved in the selection of peer reviewers for the manuscript, nor in any of the subsequent editorial decisions. Kizito Kyeremateng and Nicholas Squittieri are employees of Sun Pharmaceutical Industries, Inc.
Ethical Approval
The study was conducted in accordance with Good Clinical Practice guidelines and all local legal and regulatory requirements and was registered with ClinicalTrials.gov (NCT06415279). Institutional review board (Allendale Institutional Review Board, Old Lyme, CT) approval was obtained for the study protocol. All patients (and/or a legally acceptable representative, as applicable) were required to provide written informed consent to participate in the study.
Footnotes
Prior Publication: The data were presented as a poster at the 2024 Fall Clinical Dermatology Conference held from October 24–27, 2024, in Las Vegas, NV, USA.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen H, Zhang TC, Yin XL, Man JY, Yang XR, Lu M. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: an analysis from the Global Burden of Disease Study 2019. Br J Dermatol. 2022;186(4):673–83. [DOI] [PubMed] [Google Scholar]
- 2.Del Rosso JQ, Kircik L. The primary role of sebum in the pathophysiology of acne vulgaris and its therapeutic relevance in acne management. J Dermatolog Treat. 2024;35(1):2296855. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90(5):1006.e1-e30. [DOI] [PubMed] [Google Scholar]
- 4.Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M. Cortexolone 17alpha-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18(5):412–8. [PubMed] [Google Scholar]
- 5.Liu Y, Jiang W, Tang Y, et al. An optimal method for quantifying the facial sebum level and characterizing facial sebum features. Skin Res Technol. 2023;29(9): e13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin H, Farberg A, Frey C, et al. Unmet needs in the management of acne vulgaris: a consensus statement. J Drugs Dermatol. 2023;22(6):582–7. [DOI] [PubMed] [Google Scholar]
- 7.Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(6):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim BY, Choi JW, Park KC, Youn SW. Sebum, acne, skin elasticity, and gender difference-which is the major influencing factor for facial pores? Skin Res Technol. 2013;19(1):e45-53. [DOI] [PubMed] [Google Scholar]
- 9.Roh M, Han M, Kim D, Chung K. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155(5):890–4. [DOI] [PubMed] [Google Scholar]
- 10.Alexis A, Daniels SR, Johnson N, Pompilus F, Burgess SM, Harper JC. Development of a new patient-reported outcome measure for facial acne: the Acne Symptom and Impact Scale (ASIS). J Drugs Dermatol. 2014;13(3):333–40. [PubMed] [Google Scholar]
- 11.Arbuckle R, Atkinson MJ, Clark M, et al. Patient experiences with oily skin: the qualitative development of content for two new patient reported outcome questionnaires. Health Qual Life Outcomes. 2008;6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranzlin HT, Nap MA. The effect of a phasic oral contraceptive containing Desogestrel on seborrhea and acne. Eur J Contracept Reprod Health Care. 2006;11(1):6–13. [DOI] [PubMed] [Google Scholar]
- 13.Katz HI, Kempers S, Akin MD, Dunlap F, Whiting D, Norbart TC. Effect of a desogestrel-containing oral contraceptive on the skin. Eur J Contracept Reprod Health Care. 2000;5(4):248–55. [DOI] [PubMed] [Google Scholar]
- 14.Palombo-Kinne E, Schellschmidt I, Schumacher U, Graser T. Efficacy of a combined oral contraceptive containing 0.030 mg ethinylestradiol/2 mg dienogest for the treatment of papulopustular acne in comparison with placebo and 0.035 mg ethinylestradiol/2 mg cyproterone acetate. Contraception. 2009;79(4):282–9. [DOI] [PubMed] [Google Scholar]
- 15.Kerscher M, Reuther T, Bayrhammer J, Schramm G. Effects of an oral contraceptive containing chlormadinone and ethinylestradiol on acne-prone skin of women of different age groups: an open-label, single-centre, phase IV study. Clin Drug Investig. 2008;28(11):703–11. [DOI] [PubMed] [Google Scholar]
- 16.Gencebay G, Askin O, Serdaroglu S. Evaluation of the changes in sebum, moisturization and elasticity in acne vulgaris patients receiving systemic isotretinoin treatment. Cutan Ocul Toxicol. 2021;40(2):140–4. [DOI] [PubMed] [Google Scholar]
- 17.Aksac SE, Bilgili SG, Yavuz GO, Yavuz IH, Aksac M, Karadag AS. Evaluation of biophysical skin parameters and hair changes in patients with acne vulgaris treated with isotretinoin, and the effect of biotin use on these parameters. Int J Dermatol. 2021;60(8):980–5. [DOI] [PubMed] [Google Scholar]
- 18.Colgecen E, Ozyurt K, Ferahbas KA. The effect of systemic isotretinoin treatment on skin biophysical parameters among patients with acne vulgaris. Turk J Med Sci. 2016;46(6):1641–4. [DOI] [PubMed] [Google Scholar]
- 19.Kmiec ML, Pajor A, Broniarczyk-Dyla G. Evaluation of biophysical skin parameters and assessment of hair growth in patients with acne treated with isotretinoin. Postepy Dermatol Alergol. 2013;30(6):343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa CS, Bagatin E, Martimbianco ALC, et al. Oral isotretinoin for acne. Cochrane Database Syst Rev. 2018;11(11):CD009435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowther JM. Method for quantification of oils and sebum levels on skin using the Sebumeter((R)). Int J Cosmet Sci. 2016;38(2):210–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.